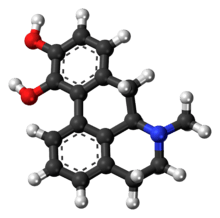
Apomorphine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Apokyn |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604020 |
| Pregnancy category | |
| Routes of administration | Oral, SC |
| ATC code | G04BE07 (WHO) N04BC07 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% following sc injection |
| Protein binding | ~50% |
| Metabolism | Hepatic |
| Biological half-life | 40 minutes (range 30-60 minutes) |
| Identifiers | |
| |
| CAS Number |
58-00-4 |
| PubChem (CID) | 6005 |
| IUPHAR/BPS | 33 |
| DrugBank |
DB00714 |
| ChemSpider |
5783 |
| UNII |
F39049Y068 |
| KEGG |
D07460 |
| ChEBI |
CHEBI:48538 |
| ChEMBL |
CHEMBL53 |
| Chemical and physical data | |
| Formula | C17H17NO2 |
| Molar mass | 267.322 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| See also: data page | |
| | |
Apomorphine (brand names Apokyn, Ixense, Spontane, Uprima) is a type of aporphine having activity as a non-selective dopamine agonist which activates both D2-like and, to an order of magnitude lesser extent, D1-like receptors.[1] It also acts as an antagonist of 5-HT2 and α-adrenergic receptors with high affinity. The compound is historically a morphine decomposition product by boiling with concentrated acid, hence the -morphine suffix. Apomorphine does not actually contain morphine or its skeleton, nor does it bind to opioid receptors. The apo- prefix relates to it being a morphine derivative ("[comes] from morphine").
Historically, apomorphine has been tried for a variety of uses including psychiatric treatment of homosexuality in the early 20th century, and more recently in treating erectile dysfunction. Currently, apomorphine is used in the treatment of Parkinson's disease. It is a potent emetic (i.e., it induces vomiting) and should not be administered without an antiemetic such as domperidone. The emetic properties of apomorphine are exploited in veterinary medicine to induce therapeutic emesis in canines that have recently ingested toxic or foreign substances.
It was also successfully used as a private treatment of heroin addiction, a purpose for which it was championed by the author William S. Burroughs. Burroughs and others claimed that it was a "metabolic regulator" with a restorative dimension to a damaged or dysfunctional dopaminergic system. There is more than enough anecdotal evidence to suggest that this offers a plausible route to an abstinence based model; however, no clinical trials have ever tested this hypothesis. A recent study indicates that apomorphine might be a suitable marker for assessing central dopamine system alterations associated with chronic heroin consumption.[2] There is, however, no clinical evidence that apomorphine is an effective and safe treatment regimen for opiate addiction. Early studies involved aversion therapy in alcoholism and anxiety, and modern reports are anecdotal, although some practitioners claimed to be using non-aversive methods.[3]
Biological Effects
Apomorphine is an agonist, or activator, of both D1 and D2 type dopamine receptors with a preference toward the D2 group. The members of the D2 subfamily, consisting of D2, D3, and D4 receptors, are involved with inhibitory neurotransmission. Inhibition is achieved through a signaling pathway in which the activated receptor, a G protein–coupled receptor, inhibits the enzyme adenylate cyclase, therefore decreasing the levels of the signaling molecule cyclic AMP.[4] The D4 receptor in particular is an important target in the signaling pathway, and is connected to several neurological disorders.[5] Shortage or excess of dopamine can prevent proper function and signaling of these receptors leading to disease states. It is classically thought that an excess of dopamine stimulation is a factor in schizophrenia, but it appears this relationship is pathway dependent and just one of several contributing factors.[6] Apomorphine is useful in diseases where dopamine levels are lower than normal, such as Parkinson’s disease, and can help to restore proper motor behavior by increasing the inhibitory signaling.[7]
Uses
Alcoholism
Apomorphine was used with some notable success as a treatment for alcohol and morphine addiction. At first this was part of a Pavlov style "conditioned-reflex" treatment, later continued with emetine by practitioners such as Voegtlin in the US, but gradually this was replaced by a recognition by various practitioners in Europe that the drug was having other effects. However, this form treatment fell out of favour, and was over by the 1980s.
Parkinson's disease
First used as a treatment for Parkinson's disease as early as 1951,[8] its clinical use was first reported in 1970 by Cotzias et al.,[9] although its emetic properties and short half-life made oral use impractical. A later study found that combining the drug with the antiemetic domperidone improved results significantly.[10] The commercialization of apomorphine for Parkinson's disease followed its successful use in patients with refractory motor fluctuations using intermittent rescue injections and continuous infusions.[11]
Therapeutic use in Parkinson's disease is effective because of the drug's strong dopaminergic action. When administered subcutaneously, apomorphine is the most effective dopamine agonist. Within 3–20 minutes of injection apomorphine demonstrates a magnitude of effect (ability to convert the patient with Parkinson's disease to the "on" state) that is comparable to L-DOPA. A single subcutaneous injection lasts for up to 90 minutes.[12] While apomorphine can be used in combination with L-DOPA, the intention is usually to reduce the L-DOPA dosing, as by this stage the patient with Parkinson's disease will probably be experiencing a great deal of dopa-induced dyskinesias and "off" periods.[12] Following a successful apomorphine challenge, training of patient and caregiver, and careful dose titration, the patient can be maintained in the "on" state by the use of an apomorphine pump as an effective monotherapy.[12]
Erectile dysfunction
Apomorphine hydrochloride (trade name "Uprima", "Ixense") was a therapy used in the treatment of erectile dysfunction (male impotence). It is its mode of stimulating dopamine in the brain which is believed to enhance the sexual response. It was found to be of poor efficacy[13] in a large-scale study by Researchers at the UK's Drug Safety Research Unit and University of Portsmouth and discontinued in the UK in January 2006.[13] Around 65-70% of doctors felt it was ineffective, with 60% of over 11,000 patients (average age 61) discontinuing in month 1 and a further 23% in month 2.[13][14] UK studies concentrated on males with generalized erectile dysfunction. Uprima affects desire and is not meant to produce a systemic effect unlike drugs such as sildenafil, which affect circulation. In those males who have problems with desire as opposed to generalized erectile dysfunction, it works as expected.
Erections in men are generally classified into two categories: Reflexogenic erections, that is erections triggered by physical stimulus of the penis, and Psychogenic erections, which are triggered by sexual fantasies, thoughts and looking at things which are sexually stimulating. Psychogenic erections are generally gradually lost in men somewhere between the ages of 45 and 65. Apomorphine has been shown to restore Psychogenic erections in men who are otherwise unable to achieve them.
Alzheimer's disease
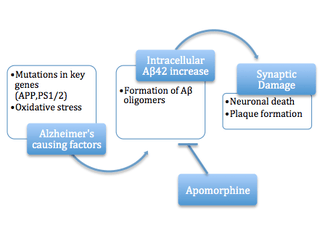
When proteins in a cell undergo misfolding due to damage, misprocessing, or mutation it can lead to the alteration of the protein’s secondary structure and change which amino acid residues are accessible. In many cases of protein aggregation, hydrophobic residues, which are normally on the interior of soluble proteins, become arranged so they are on the solvent exposed surface. This can lead to interactions between hydrophobic regions of multiple proteins, as it is entropically favorable to reduce the surface area of hydrophobicity exposed. The formation of a few molecules to form an oligomer is still soluble, but as it grows and continues to aggregate the product will fall out of solution, causing damage to the cell manifesting in proteopathic diseases. Recent studies have shown the pre-fibril aggregates are also toxic through their hydrophobic interactions with various cellular components causing cytoskeletal rearrangements and loss of cell-to-cell contacts.[16]
Apomorphine is reported to be an inhibitor of amyloid beta protein (Aβ) fiber formation and a potential therapeutic for Alzheimer's disease (AD) under the amyloid hypothesis. Apomorphine and its small molecule relatives are found to promote oligomerization of the Aβ40 group of molecules, but inhibit the more advanced fibril formation from occurring. The mechanism of protection offered by the small molecules is thought to be due to the autoxidation that occurs at the hydroxyl groups. Once this functional group was altered, the inhibitory effect could be seen to decrease, reducing either the indirect or direct interference of the fibril formation.[17]
The protective effects of apomorphine were tested in mouse models with mutations in genes related to AD, such as the amyloid precursor protein (APP) gene, which is implicated in amyloid molecule build-up. Apomorphine was seen to significantly improve memory function through the increased successful completion of the Morris Water Maze. The levels of the aberrant proteins that lead to neuronal disruption were also tested in the brains of mice. Treatment was seen to decrease the intraneuronal levels of the more aggressive Aβ42 molecule when compared to the control mice. This result is consistent with the finding that another protein linked to AD, tau protein, was seen to decrease with apomorphine treatment. Apomorphine was shown to promote Aβ degradation and inhibit formation through its anti-oxidative properties. Moderate doses were shown to provide the improvements while overuse provided no benefits and may even cause toxicity.[15]
Opioid addiction
In his Deposition: Testimony Concerning a Sickness in the introduction to later editions of Naked Lunch, William S. Burroughs wrote that apomorphine treatment was the only effective cure to opioid addiction he has encountered:
The apomorphine cure is qualitatively different from other methods of cure. I have tried them all. Short reduction, slow reduction, cortisone, antihistamines, tranquilizers, sleeping cures, tolserol, reserpine. None of these cures lasted beyond the first opportunity to relapse. I can say that I was never metabolically cured until I took the apomorphine cure... The doctor, John Yerbury Dent, explained to me that apomorphine acts on the back brain to regulate the metabolism and normalize the blood stream in such a way that the enzyme stream of addiction is destroyed over a period of four to five days. Once the back brain is regulated apomorphine can be discontinued and only used in case of relapse.
He goes on to lament the fact that as of his writing, little to no research has been done on apomorphine or variations of the drug to study its effects on curing addiction, and perhaps the possibility of retaining the positive effects while removing the side effect of vomiting.
Despite his claims throughout his life, Burroughs never really cured his addiction and was back to using opiates within years of his apomorphine "cure". However, he insisted on apomorphine’s effectiveness in several works and interviews.
Pharmacology
Apomorphine possesses affinity for the following receptors:[18]
|
It has >1,000 nM affinity for 5-HT1B, 5-HT1D, and α1A-adrenergic, and >10,000 nM affinity for β-adrenergic, H1, and mACh.[1]
Apomorphine behaves as a partial agonist at D2S (IA = 79%), D2L (IA= 53%), D3 (IA = 82%), and D4 (IA = 45%), and as an antagonist at 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, α1-adrenergic, and α2-adrenergic.[19][20] Though its efficacies at D1 and D5 are unclear, it is known to act as an agonist at these sites.[21]
Toxicity
LD50[22]
| Dose | Route of Administration | Species |
|---|---|---|
| 300 mg/kg | Oral | Mouse |
| 160 mg/kg | Intraperitoneal (ip) | Mouse |
| 56 mg/kg | Intravenous (iv) | Mouse |
Chemistry
Properties
Apomorphine is colourless as a liquid but stains green, therefore care must be taken to avoid splashes. Apomorphine does not remain stable for more than 24 hours in a plastic container, so syringes are discarded if not used within 24 hours.
Synthesis
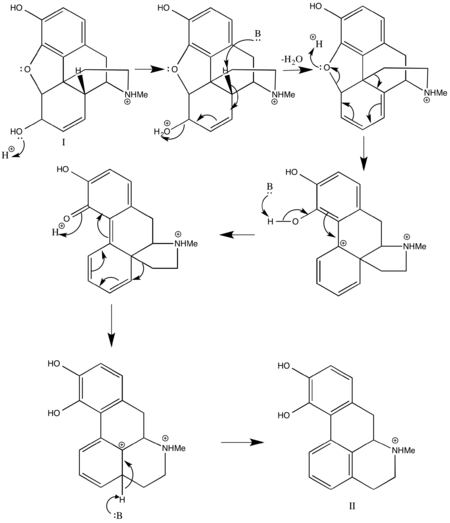
Several techniques exist for the creation of apomorphine from morphine. In the past, morphine has been combined with hydrochloric acid (HCl) at high temperatures, around 150 °C, to achieve a low yield of apomorphine which can range anywhere from 0.6% to 46%.[23]
More recent techniques create the derivative of morphine in a similar fashion, by heating it in the presence of any acid that will promote the essential dehydration rearrangement of morphine type alkaloids, such as phosphoric acid. The method then deviates by including a water scavenger, which is essential to remove the water produced by the reaction that can react with the product and lead to decreased yield. The scavenger can be any reagent that will irreversibly react with water such as phthalic anhydride or titanium chloride. The temperature required for the reaction varies based upon choice of acid and water scavenger. The yield of this reaction is much higher, and is at least 55%.[23]
In popular culture
- Apomorphine has a vital part in Agatha Christie's detective story Sad Cypress.
- The 1965 Tuli Kupferberg song "Hallucination Horrors" recommends Apomorphine at the end of each verse, as a cure for hallucinations brought on by a humorous variety of intoxicants; the song was recorded by The Fugs and appears on the album Virgin Fugs.
See also
References
- 1 2 Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666.
- ↑ Guardia J, Casas M, Prat G, Trujols J, Segura L, Sánchez-Turet M (October 2002). "The apomorphine test: a biological marker for heroin dependence disorder?". Addict Biol. 7 (4): 421–6. doi:10.1080/1355621021000006206. PMID 14578019.
- ↑ Dent JY (1949). "Apomorphine Treatment of Addiction." British Journal of Addiction to Alcohol & Other Drugs 46 (1); 15-28. doi:10.1111/j.1360-0443.1949.tb04502.x
- ↑ Neves SR, Ram PT, Iyengar R (2002). "G protein pathways". Science. 296 (5573): 1636–9. Bibcode:2002Sci...296.1636N. doi:10.1126/science.1071550. PMID 12040175.
- ↑ Ptácek R, Kuzelová H, Stefano GB (Sep 2011). "Dopamine D4 receptor gene DRD4 and its association with psychiatric disorders". Medical Science Monitor. 17 (9): RA215–RA220. doi:10.12659/MSM.881925. PMC 3560519
 . PMID 21873960.
. PMID 21873960. - ↑ Maas JW, Bowden CL, Miller AL, Javors MA, Funderburg LG, Berman N, Weintraub ST (1997). "Schizophrenia, psychosis, and cerebral spinal fluid homovanillic acid concentrations". Schizophr Bull. 23 (1): 147–54. doi:10.1093/schbul/23.1.147. PMID 9050120.
- ↑ Stacy, Mark; Silver, Dee (2008). "Apomorphine for the acute treatment of "off" episodes in Parkinson's disease". Parkinsonism & Related Disorders. 14 (2): 85–92. doi:10.1016/j.parkreldis.2007.07.016.
- ↑ Schwab R, Amador L, Lettvin J (1951). "Apomorphine in Parkinson's disease". Trans Am Neurol Assoc. 56: 251–3. PMID 14913646.
- ↑ Cotzias G, Papavasiliou P, Fehling C, Kaufman B, Mena I (1970). "Similarities between neurologic effects of L-dopa and of apomorphine". N Engl J Med. 282 (1): 31–3. doi:10.1056/NEJM197001012820107. PMID 4901383.
- ↑ Corsini G, Del Zompo M, Gessa G, Mangoni A (1979). "Therapeutic efficacy of apomorphine combined with an extracerebral inhibitor of dopamine receptors in Parkinson's disease". Lancet. 1 (8123): 954–6. doi:10.1016/S0140-6736(79)91725-2. PMID 87620.
- ↑ Stibe CM, Kempster P, Lees AJ & Stern GM (1988). "Subcutaneous apomorphine in parkinsonian on-off oscillations". Lancet. 331 (8582): 403–406. doi:10.1016/S0140-6736(88)91193-2.
- 1 2 3 Chaudhuri K, Clough C (1998). "Subcutaneous apomorphine in Parkinson's disease: Effective yet underused". BMJ. 316 (7132): 641. doi:10.1136/bmj.316.7132.641. PMC 1112674
 . PMID 9522772.
. PMID 9522772. - 1 2 3 Pharmaceutical Business Review, [ "Study shows Abbott's Uprima ineffective for most UK patients"]
- ↑ MedicineNet study review
- 1 2 Himeno, E; Ohyagi, Y; Ma, L; Nakamura, N; Miyoshi, K; Sakae, N; Motomura, K; Soejima, N; Yamasaki, R; Hashimoto, T; Tabira, T; LaFerla, FM; Kira, J (2011). "Apomorphine treatment in Alzheimer mice promoting amyloid-β degradation". Ann Neurol. 69 (2): 248–56. doi:10.1002/ana.22319. PMID 21387370.
- ↑ Zhu, YJ; Lin, H; Lal, R (2000). "Fresh and nonfibrillar amyloid beta protein(1-40) induces rapid cellular degeneration in aged human fibroblasts: evidence for AbetaP-channel-mediated cellular toxicity". FASEB J. 14 (9): 1244–54. PMID 10834946.
- ↑ Lashuel HA; Hartley DM; Balakhaneh D; Aggarwal A; Teichberg S; Callaway DJE (2002). "New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer's disease". J Biol Chem. 277 (45): 42881–42890. doi:10.1074/jbc.M206593200. PMID 12167652.
- ↑ Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 1 July 2014.
- ↑ Newman-Tancredi A, Cussac D, Audinot V, et al. (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor". The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 805–14. doi:10.1124/jpet.102.039875. PMID 12388667.
- ↑ Newman-Tancredi A, Cussac D, Quentric Y, et al. (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 815–22. doi:10.1124/jpet.102.039883. PMID 12388668.
- ↑ Hsieh GC, Hollingsworth PR, Martino B, et al. (January 2004). "Central mechanisms regulating penile erection in conscious rats: the dopaminergic systems related to the proerectile effect of apomorphine". The Journal of Pharmacology and Experimental Therapeutics. 308 (1): 330–8. doi:10.1124/jpet.103.057455. PMID 14569075.
- ↑ Lewis, R.J. Sr. (2004). Sax's Dangerous Properties of Industrial Materials. (11 ed.). Wiley, John & Sons, Incorporated. p. 287. ISBN 0471476625.
- 1 2 Narayanasamy Gurusamy. "Process for making apomorphine and apocodeine".
- ↑ Bentley, K.W. The Isoquinoline Alkaloids: A Course in Organic Chemistry. Elsevier, 2014. pp. 118–120. ISBN 1483152235.
External links
- PDSP Ki database
- "Apomorphine - Frequently Asked Questions". Britannia Pharmaceuticals.
- Andrew Lees; Kirsten Turner (2002). "Apomorphine for Parkinson's Disease" (PDF). Practical Neurology. 2 (5): 280–287. doi:10.1046/j.1474-7766.2002.00086.x. - Detailed usage guide for Apomorphine pumps for Parkinson's