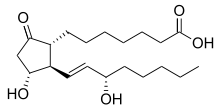
Prostaglandin E1
 | |
 | |
| Clinical data | |
|---|---|
| MedlinePlus | a695022 |
| Pregnancy category |
|
| ATC code | C01EA01 (WHO) G04BE01 (WHO) |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
745-65-3 |
| PubChem (CID) | 149351 |
| IUPHAR/BPS | 1882 |
| DrugBank |
DB00770 |
| ChEBI |
CHEBI:15544 |
| ChEMBL |
CHEMBL495 |
| ECHA InfoCard | 100.010.925 |
| Chemical and physical data | |
| Formula | C20H34O5 |
| Molar mass | 354.481 g/mol |
| | |
Prostaglandin E1 (PGE1) is a prostaglandin.
The synthetic variant is known pharmaceutically as alprostadil.[1] It is a drug used in the continuous treatment of erectile dysfunction[2] and has vasodilatory properties. Misoprostol is another synthetic prostaglandin E1 analog used to prevent gastric ulcers when taken on a continuous basis, to treat missed miscarriage, to induce labor, and to induce abortion.
Biosynthesis
Prostaglandin E1 is biosynthesized on an as-needed basis from dihomo-γ-linolenic acid (an omega-6 fatty acid) in healthy humans without coronary artery disease[3] and/or a genetic disorder.
Medical uses
Patent ductus arteriosus
Alprostadil is also used in maintaining a patent ductus arteriosus in newborns. This is primarily useful when the threat of premature closure of the ductus arteriosus exists in an infant with ductal-dependent congenital heart disease, including cyanotic lesions (e.g., hypoplastic left heart syndrome, pulmonary atresia/stenosis, tricuspid atresia/stenosis, transposition of the great arteries) and acyanotic lesions (e.g., coarctation of the aorta, critical aortic stenosis, and interrupted aortic arch).
Sexual dysfunction
Alprostadil is sold in the United States as urethral suppositories and in injectable form. The suppositories are sold under the brand name Muse.[4] The injectable forms are Edex[5] and Caverject.[6] Muse delivers alprostadil as a penile suppository, inserted into the urethra, at least ten minutes before the erection is needed. Caverject and Edex are similarly fast-acting, but instead are injected by syringe directly into the corpus cavernosum of the penis.
Alprostadil is also available as a generic. The major cost is that it must be mixed by a compounding pharmacy and supplies may be difficult to obtain. The different formulations, including Bimix and Trimix, may include papaverine and/or phentolamine. A typical mix might be 30 mg of papaverine, 2 mg of phentolamine, and 20 μg alprostadil. As a generic, it is much less expensive than the packaged injectables. It is premixed and must be kept refrigerated and the user must load a syringe with the quantity needed.
Most recently, the compound has been made easily accessible in an applicable topical cream form known as Vitaros.[7] Made by Takeda UK Ltd, it is now available in Europe and contains either 200 or 300 micrograms of alprostadil in 100 mg of cream which is directly administered as a topical cream applied to the urethra in a preloaded delivery device. The tip of the device is placed in the urethral meatus and the cream delivered into the urethra. Clinical trials for the treatment showed positive results in over 3000 men that it was tested on, and unlike other sexual dysfunction medication, it is said to be usable by men suffering from diabetes, heart problems or those who have undertaken a prostatectomy.[8] It has no known interactions with food, alcohol or other medications making it safer than other treatments containing alprostadil. Similarly to the Bimix and Trimix injections though, it must be kept under cool temperatures.
Critical limb ischemia
Alprostadil is also used for critical limb ischemia. It increases blood flow by peripheral vasodilation within five minutes and induces angiogenesis. It is most effective when the ankle pressure is at least 30 mmHg and at least one tibial artery is patent.
Adverse effects
- Accidental injury (Muse only)
- Apnea
- Bleeding:
- Cerebral
- Urethral
- Bradycardia
- Cardiac arrest
- Congestive heart failure
- Cortical proliferation of long bones
- Diarrhea
- Disseminated intravascular coagulation
- Edema
- Fever
- Flushing
- Hyperemia
- Hypotension
- Injection-site haematoma
- Injection-site ecchymosis (Caverject only)
- Pain:
- Back
- Pelvic
- Penile
- Testicular (Muse only)
- Urethral
- Prolonged erection
- Penile fibrosis
- Second-degree heart block
- Seizures
- Sepsis
- Shock
- Spasm of right ventricle infundibulum
- Supraventricular tachycardia
- Tachycardia
- Ventricular fibrillation
- Urethral burning
- Uterine rupture
References
- ↑ Cawello W, Leonhardt A, Schweer H, Seyberth HW, Bonn R, Lomeli AL (September 1995). "Dose proportional pharmacokinetics of alprostadil (prostaglandin E1) in healthy volunteers following intravenous infusion". British Journal of Clinical Pharmacology. 40 (3): 273–6. doi:10.1111/j.1365-2125.1995.tb05784.x. PMC 1365109
 . PMID 8527291.
. PMID 8527291. - ↑ Harding LM, Adeniyi A, Everson R, Barker S, Ralph DJ, Baranowski AP (December 2002). "Comparison of a needle-free high-pressure injection system with needle-tipped injection of intracavernosal alprostadil for erectile dysfunction". International Journal of Impotence Research. 14 (6): 498–501. doi:10.1038/sj.ijir.3900916. PMID 12494285.
- ↑ Stephanie M. Meller, BA, Erik Stilp, MD, Charles N. Walker, MD, Carlos Mena-Hurtado, MD (June 2013). "The Link Between Vasculogenic Erectile Dysfunction, Coronary Artery Disease, and Peripheral Artery Disease: Role of Metabolic Factors and Endovascular Therapy". Journal of Invasive Cardiology. 25 (6): 313–319.
- ↑ "Muse Suppository - Facts and Comparisons". Drugs.com. Retrieved 4 January 2013.
- ↑ Edex - Facts and Comparisons Drugs.com
- ↑ Caverject - Facts and Comparisons Drugs.com
- ↑ Vitaros 3 mg/g cream - Summary of Product Characteristics Medicines.org.uk
- ↑ Vitaros- New Erectile Dysfunction Topical Treatment Meds4All.co.uk