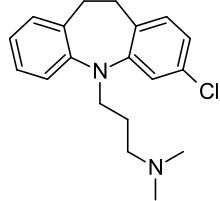
Clomipramine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anafranil, Clofranil |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697002 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Oral, IV[1] |
| ATC code | N06AA04 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 50% |
| Protein binding | 97-98% |
| Metabolism | Hepatic (CYP2D6-mediated) |
| Biological half-life | 32 hr (69 hr for active metabolite) |
| Excretion | Renal (60%), faeces (32%) |
| Identifiers | |
| |
| CAS Number |
303-49-1 |
| PubChem (CID) | 2801 |
| IUPHAR/BPS | 2398 |
| DrugBank |
DB01242 |
| ChemSpider |
2699 |
| UNII |
NUV44L116D |
| KEGG |
D07727 |
| ChEBI |
CHEBI:47780 |
| ChEMBL |
CHEMBL415 |
| ECHA InfoCard | 100.005.587 |
| Chemical and physical data | |
| Formula | C19H23ClN2 |
| Molar mass | 314.9 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Clomipramine, sold under the brand name Anafranil among others, is a tricyclic antidepressant (TCA).[2] It is used for the treatment of obsessive compulsive disorder, panic disorder, major depressive disorder, and chronic pain. It may decrease the risk of suicide in those over the age of 65. It is taken by mouth.[2]
Common side effects include dry mouth, constipation, loss of appetite, sleepiness, weight gain, sexual dysfunction, and trouble urinating. Serious side effects include an increased risk of suicidal behavior in those under the age of 25, seizures, mania, and liver problems. If stopped suddenly a withdrawal syndrome may occur with headaches, sweating, and dizziness. It is unclear if it is safe for use in pregnancy. Its mechanism of action is not entirely clear but is believed to involve increased levels of serotonin.[2]
Clomipramine was discovered in 1964 by the Swiss drug manufacturer Ciba-Geigy.[3] It is on the World Health Organization's List of Essential Medicines, the most important medication needed in a basic health system.[4] It is available as a generic medication.[2] The wholesale price in the developing world is between 0.11 and 0.21 per day as of 2014.[5] In the United States a typical dose costs about 1.20 USD per day.[2] It was made from imipramine.[3]
Medical uses
Clomipramine has a number of uses in medicine including in the treatment of:
- Obsessive–compulsive disorder (OCD) which is its only U.S. FDA-labelled indication.[6][7] Other regulatory agencies (such as the TGA of Australia and the MHRA of the UK) have also approved clomipramine for this indication.[8][9][10][11]
- Major depressive disorder (MDD) a popular off-label use in the US. It is approved by the Australian TGA and the United Kingdom MHRA for this indication.[8][9][10][11] Some have suggested the possible superior efficacy of clomipramine compared to other antidepressants in the treatment of MDD, although at the current time the evidence is insufficient to adequately substantiate this claim.[12]
- Panic disorder with or without agoraphobia.[13][14]
- Body dysmorphic disorder[15]
- Cataplexy associated with narcolepsy. Which is a TGA & MHRA-labelled indication for clomipramine.[10][11]
- Premature ejaculation[16]
- Depersonalization disorder[17]
- Chronic pain with or without organic disease, particularly headache of the tension type.[18]
- Sleep paralysis, with or without narcolepsy
- Enuresis (involuntary nightly urinating in sleep) in children and adolescents.[19]
- Trichotillomania[20][21][22]
In a meta-analysis of various trials involving fluoxetine (Prozac), fluvoxamine (Luvox), and sertraline (Zoloft) to test their relative efficacies in treating OCD, clomipramine was found to be the most effective.[23]
Pregnancy and lactation
Clomipramine use during pregnancy is associated with congenital heart defects in the newborn.[11][24] It is also associated with reversible withdrawal effects in the newborn.[25] Clomipramine is also distributed in breast milk and hence nursing while taking clomipramine is advised against.[7]
Adverse effects
Adverse effects by frequency:[6][7][8][9]
Very common (>10% frequency):
- Accommodation (eye)
- Blurred vision
- Nausea
- Dry mouth
- Constipation
- Fatigue
- Weight gain
- Increased appetite
- Dizziness
- Tremor
- Headache
- Myoclonus
- Drowsiness
- Somnolence
- Restlessness
- Micturition disorder
- Sexual dysfunction (erectile dysfunction and loss of libido)
- Hyperhidrosis (profuse sweating)
Common (1-10% frequency):
- Weight loss
- Orthostatic hypotension
- Sinus tachycardia
- Clinically irrelevant ECG changes (e.g. T- and ST-wave changes) in patients of normal cardiac status
- Palpitations
- Tinnitus (hearing ringing in one's ears)
- Mydriasis (dilated pupils)
- Vomiting
- Abdominal disorders
- Diarrhoea
- Decreased appetite
- Transaminases increased
- Alkaline phosphatase increased
- Speech disorders
- Paraesthesia
- Muscle hypertonia
- Dysgeusia
- Memory impairment
- Muscular weakness
- Disturbance in attention
- Confusional state
- Disorientation
- Hallucinations (particularly in elderly patients and patients with Parkinson's disease)
- Anxiety
- Agitation
- Sleep disorders
- Mania
- Hypomania
- Aggression
- Depersonalisation
- Insomnia
- Nightmares
- Aggravation of depression
- Delirium
- Galactorrhoea (lactation that is not associated with pregnancy or breastfeeding)
- Breast enlargement
- Yawning
- Hot flush
- Dermatitis allergic (skin rash, urticaria)
- Photosensitivity reaction
- Pruritus (itching)
Uncommon (0.1-1% frequency):
- Convulsions
- Ataxia
- Arrhythmias
- Elevated blood pressure
- Activation of psychotic symptoms
Very rare (<0.01% frequency):
- Pancytopaenia — an abnormally low amount of all the different types of blood cells in the blood (including platelets, white blood cells and red blood cells).
- Leucopenia — a low white blood cell count.
- Agranulocytosis — basically a worse form of leucopaenia; a dangerously low white blood cell count which leaves one open to life-threatening infections due to the role of the white blood cells in defending the body from invaders.
- Thrombocytopenia — an abnormally low amount of platelets in the blood which are essential to clotting and hence this leads to an increased tendency to bruise and bleed, including, potentially, internally.
- Eosinophilia — an abnormally high number of eosinophils — the cells that fight off parasitic infections — in the blood.
- Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) — a potentially fatal reaction to certain medications that is due to an excessive release of antidiuretic hormone — a hormone that prevents the production of urine by increasing the reabsorption of fluids in the kidney — this results in the development of various electrolyte abnormalities (e.g. hyponatraemia [low blood sodium], hypokalaemia [low blood potassium], hypocalcaemia [low blood calcium]).
- Glaucoma
- Oedema (local or generalised)
- Alopecia (hair loss)
- Hyperpyrexia (a high fever that is above 41.5 °C)
- Hepatitis (liver swelling) with or without jaundice — the yellowing of the eyes, the skin, and mucous membranes due to impaired liver function.
- Abnormal ECG
- Anaphylactic and anaphylactoid reactions including hypotension
- Neuroleptic malignant syndrome (NMS) — a potentially fatal side effect of antidopaminergic agents such as antipsychotics, tricyclic antidepressants and antiemetics (drugs that relieve nausea and vomiting). NMS develops over a period of days or weeks and is characterised by the following symptoms:
- Tremor
- Muscle rigidity
- Mental status change (such as confusion, delirium, mania, hypomania, agitation, coma, etc.)
- Hyperthermia (high body temperature)
- Tachycardia (high heart rate)
- Blood pressure changes
- Diaphoresis (sweating profusely)
- Diarrhoea
- Alveolitis allergic (pneumonitis) with or without eosinophilia
- Purpura
- Conduction disorder (e.g. widening of QRS complex, prolonged QT interval, PQ changes, bundle-branch block, torsade de pointes, particularly in patients with hypokalaemia)
Withdrawal
Withdrawal symptoms may occur during gradual or particularly abrupt withdrawal of tricyclic antidepressant drugs. Possible symptoms include: nausea, vomiting, abdominal pain, diarrhoea, insomnia, headache, nervousness, anxiety, dizziness and worsening of psychiatric status.[8] Differentiating between the return of the original psychiatric disorder and clomipramine withdrawal symptoms is important.[26] Clomipramine withdrawal can be severe.[27] Withdrawal symptoms can also occur in neonates when clomipramine is used during pregnancy.[25] A major mechanism of withdrawal from tricyclic antidepressants is believed to be due to a rebound effect of excessive cholinergic activity due to neuroadaptations as a result of chronic inhibition of cholinergic receptors by tricyclic antidepressants. Restarting the antidepressant and slow tapering is the treatment of choice for tricyclic antidepressant withdrawal. Some withdrawal symptoms may respond to anticholinergics, such as atropine or benztropine mesylate.[28]
Drug interactions
Clomipramine may interact with a number of different medications, including the monoamine oxidase inhibitors which include isocarboxazid, moclobemide, phenelzine, selegiline and tranylcypromine, antiarrhythmic agents (due to the effects of TCAs like clomipramine on cardiac conduction. There is also a potential pharmacokinetic interaction with quinidine due to the fact that clomipramine is metabolised by CYP2D6 in vivo), diuretics (due to the potential for hypokalaemia (low blood potassium) to develop which increases the risk for QT interval prolongation and torsades de pointes), the selective serotonin reuptake inhibitors (SSRIs; due to both potential additive serotonergic effects leading to serotonin syndrome and the potential for a pharmacokinetic interaction with the SSRIs that inhibit CYP2D6 [e.g. fluoxetine and paroxetine]) and serotonergic agents such as triptans, other tricyclic antidepressants, tramadol, etc. (due to the potential for serotonin syndrome).[8] Its use is also advised against in those concurrently on CYP2D6 inhibitors due the potential for increased plasma levels of clomipramine and the resulting potential for CNS and cardiotoxicity.[8]
Contraindications
Contraindications include:[9]
- Known hypersensitivity to clomipramine, or any of the excipients or cross-sensitivity to tricyclic antidepressants of the dibenzazepine group
- Recent myocardial infarction
- Any degree of heart block or other cardiac arrhythmias
- Mania
- Severe liver disease
- Narrow angle glaucoma
- Urinary retention
- It must not be given in combination or within 3 weeks before or after treatment with an monoamine oxidase inhibitor. (moclobemide included, however, Clomipramine can be initiated 48 hours upon discontinuation of Moclobemide)
Overdose
Clomipramine overdose usually presents with the following symptoms:[6][8][9]
- Signs of central nervous system depression such as:
- stupor
- coma
- drowsiness
- restlessness
- ataxia
- Mydriasis
- Convulsions
- Enhanced reflexes
- Muscle rigidity
- Athetoid and choreoathetoid movements
- Serotonin syndrome - a condition with many of the same symptoms as neuroleptic malignant syndrome but has a significantly more rapid onset
- Cardiovascular effects including:
- arrhythmias (including Torsades de pointes)
- tachycardia
- QTc interval prolongation
- conduction disorders
- hypotension
- shock
- heart failure
- cardiac arrest
- Apnoea
- Cyanosis
- Respiratory depression
- Vomiting
- Fever
- Sweating
- Oliguria
- Anuria
There is no specific antidote for overdose and all treatment is purely supportive and symptomatic.[8] Treatment with activated charcoal may be used to limit absorption in cases of oral overdose.[8] Anyone suspected of overdosing on clomipramine should be hospitalised and kept under close surveillance for at least 72 hours.[8] Clomipramine has been reported as being less toxic in overdose than most other TCAs in one meta-analysis but this may well be due to the circumstances surrounding most overdoses as clomipramine is more frequently used to treat conditions for which the rate of suicide is not particularly high such as obsessive-compulsive disorder.[29] In another meta-analysis, however, clomipramine was associated with a significant degree of toxicity in overdose.[30]
Mechanism of action
Clomipramine is a highly selective (~200x over norepinephrine) inhibitor of serotonin reuptake.[31] It is also an antagonist/inverse agonist at the histamine H1 receptor, the muscarinic acetylcholine receptors and the α1 adrenergic receptor.[31] These last three actions likely contributes to its adverse effects.[31]
Clomipramine's binding profile is as follows:[32][33][34][35][36]
| Biological protein | SERT | NET | DAT | 5-HT1A | 5-HT2A | 5-HT2C | 5-HT3 | 5-HT6 | 5-HT7 | α1A | α2A | D1 | D2 | D3 | H1 | mAChRs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki (nM) | 0.21 | 45.85 | 2,605 | >10,000 | 35.5 | 64.6 | 85.1 | 53.8 | 127 | 3.2 | 525 | 219 | 119.8 | 40.05 | 31.2 | 37 |
In addition clomipramine's active metabolite desmethylclomipramine is known to display the following affinity:
- Norepinephrine transporter (NET) (Ki = <1 nM)[37]
Pharmacokinetics
Peak plasma concentrations occur around 2–6 hours (with an average of 4.7 hours) after taking clomipramine orally.[6] Maximum plasma concentrations of clomipramine are around 56-154 ng/mL.[6] Steady state concentrations of clomipramine are around 134-532 ng/mL (with an average of 218 ng/mL) and are reached after 7–14 days of repeated dosing.[6] Steady-state concentration of the active metabolite, desmethylclomipramine, are around 230-550 ng/mL.[6] Its oral bioavailability is 50%.[6] It binds approximately 97-98% to plasma proteins,[6][7] primarily albumin.[6] It is metabolised in the liver primarily by CYP2D6.[7] It has an elimination half-life of 32 hours, and its N-desmethyl metabolite, desmethylclomipramine, has a half-life of approximately 69 hours.[7] It is mostly excreted in urine (60%) and faeces (32%).[7] Its volume of distribution (Vd) is approximately 17 L/kg.[7]
Veterinary uses
In the U.S., clomipramine is only licensed to treat separation anxiety in dogs for which it is sold under the brand name Clomicalm.[38] It has proven effective in the treatment of obsessive-compulsive disorders in cats and dogs.[39][40] In dogs, it has also demonstrated similar efficacy to fluoxetine in treating tail chasing.[41] In dogs some evidence suggests its efficacy in treating noise phobia.[42]
Clomipramine has also demonstrated efficacy in treating urine spraying in cats.[43] Various studies have been done on the effects of clomipramine on cats to reduce urine spraying/marking behavior. It has been shown to be able to reduce this behavior by up to 75% reduction of the behavior in a trial period of four weeks.[44]
References
- ↑ Taylor, D; Paton, C; Shitij, K (2012). Maudsley Prescribing Guidelines in Psychiatry (11th ed.). West Sussex: Wiley-Blackwell. ISBN 978-0-47-097948-8.
- 1 2 3 4 5 "Clomipramine Hydrochloride". The American Society of Health-System Pharmacists. Retrieved Dec 2, 2015.
- 1 2 Zohar, Joseph (2012). Obsessive-compulsive disorder current science and clinical practice. Chichester, West Sussex, UK: Wiley-Blackwell. p. 32. ISBN 9781118308011.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ "Clomipramine". International Drug Price Indicator Guide. Retrieved 3 December 2015.
- 1 2 3 4 5 6 7 8 9 10 "ANAFRANIL (CLOMIPRAMINE HYDROCHLORIDE) CAPSULE [MALLINCKRODT, INC.]". DailyMed. Mallinckrodt, Inc. October 2012. Retrieved 30 November 2013.
- 1 2 3 4 5 6 7 8 "Anafranil (clomipramine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 30 November 2013.
- 1 2 3 4 5 6 7 8 9 10 "ANAFRANIL® (clomipramine)" (PDF). TGA eBusiness Services. NOVARTIS Pharmaceuticals Australia Pty Limited. 7 December 2012. Retrieved 30 November 2013.
- 1 2 3 4 5 "Anafranil 75mg SR Tablets - Summary of Product Characteristics (SPC) - (eMC)". electronic Medicines Compendium. Novartis Pharmaceuticals UK Ltd. 8 October 2012. Retrieved 2 July 2014.
- 1 2 3 Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8.
- 1 2 3 4 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ Montgomery, SA; Baldwin, DS; Blier, P; Fineberg, NA; Kasper, S; Lader, M; Lam, RW; Lépine, JP; Möller, HJ; Nutt, DJ; Rouillon, F; Schatzberg, AF; Thase, ME (November 2007). "Which antidepressants have demonstrated superior efficacy? A review of the evidence". International Clinical Psychopharmacology. 22 (6): 323–329. doi:10.1097/YIC.0b013e3282eff7e0. PMID 17917550.
- ↑ Saeed, SA; Bruce, TJ (May 1998). "Panic Disorder: Effective Treatment Options". American Family Physician. 57 (10): 2405–2412.
- ↑ Papp, LA; Schneier, FR; Fyer, AJ; Leibowitz, MR; Gorman, JM; Coplan, JD; Campeas, R; Fallon, BA; Klein, DF (October 1997). "Clomipramine treatment of panic disorder: pros and cons". Journal of Clinical Psychiatry. 58 (10): 423–425. doi:10.4088/JCP.v58n1002. PMID 9375591.
- ↑ Hollander, E; Allen, A; Kwon, J; Aronowitz, B; Schmeidler, J; Wong, C; Simeon, D (November 1999). "Clomipramine vs desipramine crossover trial in body dysmorphic disorder: selective efficacy of a serotonin reuptake inhibitor in imagined ugliness" (PDF). Archives of General Psychiatry. 56 (11): 1033–1039. doi:10.1001/archpsyc.56.11.1033. PMID 10565503.
- ↑ Palmer, NR; Stuckey, BG (June 2008). "Premature ejaculation: a clinical update" (PDF). The Medical Journal of Australia. 188 (11): 662–666. PMID 18513177.
- ↑ Hollander, E.; Hwang; Mullen (1993). "Clinical and research issues in depersonalization syndrome.". Psychosomatics. 34: 193–194. doi:10.1016/s0033-3182(93)71919-2.
- ↑ Nilsson, HL; Knorring, LV. "Review. Clomipramine in acute and chronic pain syndromes". Nordic Journal of Psychiatry. 43 (s20): 101–113. doi:10.3109/08039488909100841.
- ↑ Glazener, CMA; Evans, JHC; Peto, RE (2003). "Tricyclic and related drugs for nocturnal enuresis in children" (PDF). The Cochrane Database of Systematic Reviews (3): CD002117. doi:10.1002/14651858.CD002117. PMID 12917922.
- ↑ Rothbart, R; Amos, T; Siegfried, N; Ipser, JC; Fineberg, N; Chamberlain, SR; Stein, DJ (November 2013). "Pharmacotherapy for trichotillomania" (PDF). The Cochrane Database of Systematic Reviews. 11: CD007662. doi:10.1002/14651858.CD007662.pub2.
- ↑ "Trichotillomania Medication". Medscape Reference. WebMD. 10 October 2013. Retrieved 1 December 2013.
- ↑ Franklin, ME; Zagrabbe, K; Benavides, KL (August 2011). "Trichotillomania and its treatment: a review and recommendations". Expert Review of Neurotherapeutics. 11 (8): 1165–1174. doi:10.1586/ern.11.93. PMC 3190970
 . PMID 21797657.
. PMID 21797657. - ↑ Greist, JH (Jan 1995). "Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis". Archives of General Psychiatry. 52 (1): 53–60. doi:10.1001/archpsyc.1995.03950130053006. PMID 7811162.
- ↑ Källén, B; Otterblad Olausson, P (Apr 2006). "Antidepressant drugs during pregnancy and infant congenital heart defect". Reproductive Toxicology. 21 (3): 221–222. doi:10.1016/j.reprotox.2005.11.006. PMID 16406480.
- 1 2 Bloem, BR.; Lammers, GJ.; Roofthooft, DW.; De Beaufort, AJ.; Brouwer, OF. (Jul 1999). "Clomipramine withdrawal in newborns." (PDF). Arch Dis Child Fetal Neonatal Ed. 81 (1): F77. doi:10.1136/fn.81.1.f77a. PMC 1720967
 . PMID 10744432.
. PMID 10744432. - ↑ Zemishlany, Z.; Aizenberg, D.; Hermesh, H.; Weizman, A. (Oct 1992). "[Withdrawal reactions after clomipramine].". Harefuah. 123 (7-8): 252–5, 307. PMID 1459499.
- ↑ Kraft, TB. (Aug 1977). "[Severe withdrawal symptoms following use of clomipramine].". Ned Tijdschr Geneeskd. 121 (33): 1293. PMID 895917.
- ↑ Wolfe, RM. (Aug 1997). "Antidepressant withdrawal reactions.". Am Fam Physician. 56 (2): 455–62. PMID 9262526.
- ↑ White, N; Litovitz, T; Clancy, C (December 2008). "Suicidal antidepressant overdoses: a comparative analysis by antidepressant type" (PDF). Journal of Medical Toxicology. 4 (4): 238–250. doi:10.1007/BF03161207. PMC 3550116
 . PMID 19031375.
. PMID 19031375. - ↑ Hawton, K.; Bergen, H.; Simkin, S.; Cooper, J.; Waters, K.; Gunnell, D.; Kapur, N. (May 2010). "Toxicity of antidepressants: rates of suicide relative to prescribing and non-fatal overdose" (PDF). The British Journal of Psychiatry. 196 (5): 354–358. doi:10.1192/bjp.bp.109.070219. PMC 2862059
 . PMID 20435959.
. PMID 20435959. - 1 2 3 Brunton, L; Chabner, B; Knollman, B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.
- ↑ Tatsumi M, Groshan K, Blakely RD, Richelson E (December 1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". European Journal of Pharmacology. 340 (2–3): 249–58. doi:10.1016/S0014-2999(97)01393-9. PMID 9537821.
- ↑ Cusack B, Nelson A, Richelson E (May 1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology. 114 (4): 559–65. doi:10.1007/BF02244985. PMID 7855217.
- ↑ Wander TJ, Nelson A, Okazaki H, Richelson E (December 1986). "Antagonism by antidepressants of serotonin S1 and S2 receptors of normal human brain in vitro". European Journal of Pharmacology. 132 (2–3): 115–21. doi:10.1016/0014-2999(86)90596-0. PMID 3816971.
- ↑ Richelson E, Nelson A (July 1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". The Journal of Pharmacology and Experimental Therapeutics. 230 (1): 94–102. PMID 6086881.
- ↑ Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 30 November 2013.
- ↑ P K Gillman (July 2007). "Tricyclic antidepressant pharmacology and therapeutic drug interactions updated". Br J Pharmacol. 151 (6): 737–748. doi:10.1038/sj.bjp.0707253. PMC 2014120
 . PMID 17471183.
. PMID 17471183. - ↑ "CLOMICALM clomipramine tablet CLOMIPRAMINE- clomipramine powder Novartis Animal Health US, Inc.". Drugs@FDA. Novartis Animal Health US, Inc. December 1998. Retrieved 1 December 2013.
- ↑ Seksel, K; Lindeman, MJ (May 1998). "Use of clomipramine in the treatment of anxiety-related and obsessive-compulsive disorders in cats". Australian Veterinary Journal. 76 (5): 317–321. doi:10.1111/j.1751-0813.1998.tb12353.x. PMID 9631696.
- ↑ Overall, KL; Dunham, AE (November 2002). "Clinical features and outcome in dogs and cats with obsessive-compulsive disorder: 126 cases (1989-2000)" (PDF). Journal of the American Veterinary Medical Association. 221 (10): 1445–1452. doi:10.2460/javma.2002.221.1445. PMID 12458615.
- ↑ Yalcin, E (2010). "Comparison of clomipramine and fluoxetine treatment of dogs with tail chasing" (PDF). Tierarztl Prax Ausg K Kleintiere Heimtiere. 38 (5): 295–299. PMID 22215314.
- ↑ Seksel, K; Lindeman, MJ (April 2001). "Use of clomipramine in treatment of obsessive-compulsive disorder, separation anxiety and noise phobia in dogs: a preliminary, clinical study". Australian Veterinary Journal. 79 (4): 252–256. doi:10.1111/j.1751-0813.2001.tb11976.x. PMID 11349411.
- ↑ King, JN; Steffan, J; Heath, SE; Simpson, BS; Crowell-Davis, SL; Harrington, LJ; Weiss, AB; Seewald, W (September 2004). "Determination of the dosage of clomipramine for the treatment of urine spraying in cats". Journal of the American Veterinary Medical Association. 225 (6): 881–887. doi:10.2460/javma.2004.225.881.
- ↑ Landsberg, G.M.; Wilson, A.L. (2005). "Effects of clomipramine on cats presented for urine marking". J. Am. Anim. Hosp. Assoc. 41: 3–11. doi:10.5326/0410003.