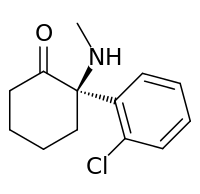
Arketamine
Arketamine, also (R)-ketamine or (R)-(−)-ketamine, is the (R)-(−) enantiomer of ketamine.[1][2][3][4] Similarly to racemic ketamine and esketamine, the S(+) enantiomer of ketamine, arketamine is biologically active; however, it is less potent as an NMDA receptor antagonist and anesthetic and thus has never been approved or marketed for clinical use as an enantiopure drug.[1][4] On the other hand, it appears to be more effective as an antidepressant.[5]
Relative to esketamine, arketamine possesses 4–5 times lower affinity for the PCP site of the NMDA receptor.[2][3][6] In accordance, arketamine is significantly less potent than racemic ketamine and especially esketamine in terms of anesthetic, analgesic, and sedative-hypnotic effects.[2][6] Racemic ketamine has weak affinity for the sigma receptor, where it acts as an agonist, whereas esketamine binds negligibly to this receptor, and so the sigma receptor activity of racemic ketamine lies in arketamine.[7] It has been suggested that this action of arketamine may play a role in the hallucinogenic effects of racemic ketamine and that it may be responsible for the lowering of the seizure threshold seen with racemic ketamine.[2][7] Esketamine inhibits the dopamine transporter about 8-fold more potently than does arketamine, and so is about 8 times more potent as a dopamine reuptake inhibitor.[8] Arketamine and esketamine possess similar potency for interaction with the muscarinic acetylcholine receptors.[9]
Paradoxically, arketamine shows greater and longer-lasting rapid antidepressant effects in animal models of depression relative to esketamine.[5][10][11] It has been suggested that this difference may have due to with the possibility of different activity of arketamine and esketamine and their respective metabolites at the α7-nicotinic receptor, as norketamine and hydroxynorketamine are potent antagonists of this receptor and markers of potential rapid antidepressant effects (specifically, increased mammalian target of rapamycin function) correlate closely with their affinity for it.[12][13][14] The picture is unclear however, and other mechanisms have also been implicated.[11]
In rodent studies, esketamine produced hyperlocomotion, prepulse inhibition deficits, and rewarding effects, while arketamine did not, in accordance with its lower potency as an NMDA receptor antagonist and dopamine reuptake inhibitor.[11] As such, arketamine may have a lower propensity for producing psychotomimetic effects and a lower abuse potential in addition to superior antidepressant efficacy.[11]
References
- 1 2 C.R. Ganellin; David J. Triggle (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1188–. ISBN 978-0-412-46630-4.
- 1 2 3 4 John D. Current, M.D. Pharmacology for Anesthetists. PediaPress. pp. 263–. GGKEY:6RRHEC392UN.
- 1 2 David T. Yew (6 March 2015). Ketamine: Use and Abuse. Taylor & Francis. pp. 269–. ISBN 978-1-4665-8340-5.
- 1 2 Singh, Jaskaran B.; Fedgchin, Maggie; Daly, Ella; Xi, Liwen; Melman, Caroline; De Bruecker, Geert; Tadic, Andre; Sienaert, Pascal; Wiegand, Frank; Manji, Husseini; Drevets, Wayne C.; Van Nueten, Luc (2015). "Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study". Biological Psychiatry. doi:10.1016/j.biopsych.2015.10.018. ISSN 0006-3223.
- 1 2 Zhang JC, Li SX, Hashimoto K (2014). "R (-)-ketamine shows greater potency and longer-lasting antidepressant effects than S (+)-ketamine". Pharmacol. Biochem. Behav. 116: 137–41. doi:10.1016/j.pbb.2013.11.033. PMID 24316345.
- 1 2 Paul G. Barash; Bruce F. Cullen; Robert K. Stoelting; Michael Cahalan; M. Christine Stock (28 March 2012). Clinical Anesthesia. Lippincott Williams & Wilkins. pp. 456–. ISBN 978-1-4511-4795-7.
- 1 2 Joris C. Verster; Kathleen Brady; Marc Galanter; Patricia Conrod (6 July 2012). Drug Abuse and Addiction in Medical Illness: Causes, Consequences and Treatment. Springer Science & Business Media. pp. 205–. ISBN 978-1-4614-3375-0.
- ↑ Nishimura M, Sato K (1999). "Ketamine stereoselectively inhibits rat dopamine transporter". Neurosci. Lett. 274 (2): 131–4. doi:10.1016/s0304-3940(99)00688-6. PMID 10553955.
- ↑ J. Vuyk; Stefan Schraag (6 December 2012). Advances in Modelling and Clinical Application of Intravenous Anaesthesia. Springer Science & Business Media. pp. 270–. ISBN 978-1-4419-9192-8.
- ↑ Hashimoto, Kenji (2014). "The R-Stereoisomer of Ketamine as an Alternative for Ketamine for Treatment-resistant Major Depression". Clinical Psychopharmacology and Neuroscience. 12 (1): 72–73. doi:10.9758/cpn.2014.12.1.72. ISSN 1738-1088.
- 1 2 3 4 Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015). "R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects". Transl Psychiatry. 5: e632. doi:10.1038/tp.2015.136. PMID 26327690.
- ↑ van Velzen, Monique; Dahan, Albert (2014). "Ketamine Metabolomics in the Treatment of Major Depression". Anesthesiology. 121 (1): 4–5. doi:10.1097/ALN.0000000000000286. ISSN 0003-3022.
- ↑ Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O'Loughlin K, Torjman MC, Bernier M, Wainer IW (2014). "(R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function". Anesthesiology. 121 (1): 149–59. doi:10.1097/ALN.0000000000000285. PMID 24936922.
- ↑ Singh NS, Zarate CA, Moaddel R, Bernier M, Wainer IW (2014). "What is hydroxynorketamine and what can it bring to neurotherapeutics?". Expert Rev Neurother. 14 (11): 1239–42. doi:10.1586/14737175.2014.971760. PMID 25331415.
|
|---|
|
Psychedelics
(5-HT2A
agonists) | | |
|---|
| | | |
|---|
| HOT-* | |
|---|
| 25*-NB*
(excludes FLY) | |
|---|
| Subst.
mescaline | |
|---|
| | |
|---|
| 3C-* | |
|---|
| 4C-* | |
|---|
| FLY | |
|---|
| | |
|---|
| Others | |
|---|
|
|---|
| | |
|---|
| | | |
|---|
| |
- 4,5-DHP-DMT
- 2,N,N-TMT
- 4-AcO-DMT
- 4-HO-5-MeO-DMT
- 4,N,N-TMT
- 4-Propionyloxy-DMT
- 5,6-diBr-DMT
- 5-AcO-DMT
- 5-Bromo-DMT
- 5-MeO-2,N,N-TMT
- 5-MeO-4,N,N-TMT
- 5-MeO-α,N,N-TMT
- 5-MeO-DMT
- 5-N,N-TMT
- 7,N,N-TMT
- α,N,N-TMT
- (Bufotenin) 5-HO-DMT
- DMT
- Norbaeocystin
- (Psilocin) 4-HO-DMT
- (Psilocybin) 4-PO-DMT
|
|---|
| | |
|---|
| | |
|---|
| | |
|---|
| | |
|---|
| | |
|---|
| | |
|---|
| | |
|---|
| Others | |
|---|
|
|---|
| Others | |
|---|
|
|---|
|
Dissociatives
(NMDAR
antagonists) | |
|---|
|
Deliriants
(mAChR
antagonists) | |
|---|
|
| Others | |
|---|
|
|---|
|
|
|
|
|
| |
|---|
| Non-selective | |
|---|
| MAOA-selective | |
|---|
| MAOB-Selective | |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| |
|---|
| | mACh |
- Muscarinic antagonists: 3-Quinuclidinyl benzilate
- 4-DAMP
- Aclidinium bromide
- Anisodamine
- Anisodine
- Antihistamines (first-generation) (e.g., brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine (pyrilamine), phenindamine, pheniramine, promethazine, tripelennamine, triprolidine)
- Atropine
- Atropine methonitrate
- Atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, zotepine)
- Benactyzine
- Benzatropine (benztropine)
- Benzilylcholine mustard
- Benzydamine
- BIBN 99
- Biperiden
- Bornaprine
- CAR-226,086
- CAR-301,060
- CAR-302,196
- CAR-302,282
- CAR-302,368
- CAR-302,537
- CAR-302,668
- Caramiphen
- Cloperastine
- CS-27349
- Cyclobenzaprine
- Cyclopentolate
- Darifenacin
- DAU-5884
- Dimethindene
- Dexetimide
- DIBD
- Dicyclomine (dicycloverine)
- Ditran
- EA-3167
- EA-3443
- EA-3580
- EA-3834
- Etanautine
- Etybenzatropine (ethybenztropine)
- Flavoxate
- Himbacine
- HL-031,120
- Ipratropium bromide
- J-104,129
- Hyoscyamine
- Mamba toxin 3
- Mamba toxin 7
- Mazaticol
- Mebeverine
- Methoctramine
- Metixene
- N-Ethyl-3-piperidyl benzilate
- N-Methyl-3-piperidyl benzilate
- Orphenadrine
- Otenzepad
- Oxybutynin
- PBID
- PD-102,807
- PD-0298029
- Phenglutarimide
- Phenyltoloxamine
- Pipenzolate bromide
- Pirenzepine
- Piroheptine
- Procyclidine
- Profenamine
- Revefenacin
- RU-47,213
- SCH-57,790
- SCH-72,788
- SCH-217,443
- Scopolamine (hyoscine)
- Sofpironium bromide
- Solifenacin
- Telenzepine
- Tetracyclic antidepressants (e.g., amoxapine, maprotiline, mianserin, mirtazapine)
- Timepidium bromide
- Tiotropium bromide
- Tolterodine
- Tricyclic antidepressants (e.g., amitriptyline, butriptyline, clomipramine, desipramine, dosulepin (dothiepin), doxepin, imipramine, lofepramine, nortriptyline, protriptyline, trimipramine)
- Trihexyphenidyl
- Tripitamine
- Tropacine
- Tropatepine
- Tropicamide
- Typical antipsychotics (e.g., chlorpromazine, loxapine, thioridazine)
- WIN-2299
- Xanomeline
- Zamifenacin
|
|---|
| | nACh |
- Nicotinic agonists: 5-HIAA
- A-84,543
- A-366,833
- A-582,941
- A-867,744
- ABT-202
- ABT-418
- ABT-560
- ABT-894
- Acetylcholine
- Altinicline
- Anabasine
- Anatoxin-a
- AR-R17779
- Butinoline
- Butyrylcholine
- Carbachol
- Choline
- Cotinine
- Cytisine
- Decamethonium
- Desformylflustrabromine
- Dianicline
- Dimethylphenylpiperazinium
- Epibatidine
- Epiboxidine
- Ethanol
- Ethoxysebacylcholine
- EVP-4473
- EVP-6124
- Galantamine
- GTS-21
- Ispronicline
- Ivermectin
- Levamisole
- Lobeline
- MEM-63,908 (RG-3487)
- Morantel
- Nicotine (tobacco)
- NS-1738
- PHA-543,613
- PHA-709,829
- PNU-120,596
- PNU-282,987
- Pozanicline
- Rivanicline
- RJR-2429
- Sazetidine A
- SB-206553
- Sebacylcholine
- SIB-1508Y
- SIB-1553A
- SSR-180,711
- Suberyldicholine
- Suxamethonium (succinylcholine)
- TC-1698
- TC-1734
- TC-1827
- TC-2216
- TC-5214
- TC-5619
- TC-6683
- Tebanicline
- Tropisetron
- UB-165
- Varenicline
- WAY-317,538
- XY-4083
|
|---|
|
|
|
|
|
| |
|---|
| | ChAT |
- Inhibitors: 1-(-Benzoylethyl)pyridinium
- 2-(α-Naphthoyl)ethyltrimethylammonium
- 3-Chloro-4-stillbazole
- 4-(1-Naphthylvinyl)pyridine
- Acetylseco hemicholinium-3
- Acryloylcholine
- AF64A
- B115
- BETA
- CM-54,903
- N,N-Dimethylaminoethylacrylate
- N,N-Dimethylaminoethylchloroacetate
|
|---|
| | AChE | |
|---|
| | BChE |
- Inhibitors: Cymserine
- Many of the AChE inhibitors listed above
|
|---|
|
|
|
|
|
|
|
|---|
|
| D1-like | |
|---|
|
| D2-like | |
|---|
|
- See also: Adrenergics
- Melatonergics
- Serotonergics
- Monoamine reuptake and release modulators
- Monoamine metabolism modulators
- Monoamine neurotoxins
|
|
|---|
|
Receptor
(ligands) | | AMPA | |
|---|
| | NMDA |
- Antagonists: Competitive antagonists: AP5 (APV)
- AP7
- CGP-37849
- CGP-39551
- CGP-39653
- CGP-40116
- CGS-19755
- CPP
- LY-233,053
- LY-235,959
- LY-274,614
- MDL-100,453
- Midafotel (d-CPPene)
- NPC-12,626
- NPC-17,742
- PBPD
- PEAQX
- Perzinfotel
- PPDA
- SDZ-220581
- Selfotel; Noncompetitive antagonists: ARR-15,896
- Caroverine
- Dexanabinol
- FPL-12495
- FR-115,427
- Hodgkinsine
- Magnesium
- MDL-27,266
- NPS-1506
- Psychotridine
- Zinc; Uncompetitive pore blockers: 2-MDP
- 3-HO-PCP
- 3-MeO-PCE
- 3-MeO-PCMo
- 3-MeO-PCP
- 4-MeO-PCP
- 8A-PDHQ
- 18-MC
- α-Endopsychosin
- Alaproclate
- Amantadine
- Aptiganel
- Arketamine
- ARL-12,495
- ARL-15,896-AR
- ARL-16,247
- Budipine
- Conaridine
- Delucemine
- Dexoxadrol
- Dextrallorphan
- Dieticyclidine
- Diphenidine
- Dizocilpine
- Ephenidine
- Esketamine
- Etoxadrol
- Eticyclidine
- Fluorolintane
- Gacyclidine
- Ibogaine
- Ibogamine
- Indantadol
- Ketamine
- Ketobemidone
- Lanicemine
- Loperamide
- Memantine
- Methadone (Levomethadone)
- Methorphan (Dextromethorphan
- Levomethorphan)
- Methoxetamine
- Methoxphenidine
- Milnacipran
- Morphanol (Dextrorphan
- Levorphanol)
- NEFA
- Neramexane
- Nitromemantine
- Nitrous oxide
- Noribogaine
- Norketamine
- Orphenadrine
- PCPr
- Pethidine (meperidine)
- Phencyclamine
- Phencyclidine
- Propoxyphene
- Remacemide
- Rhynchophylline
- Rimantadine
- Rolicyclidine
- Sabeluzole
- Tabernanthine
- Tenocyclidine
- Tiletamine
- Tramadol
- Xenon; Glycine site antagonists: 4-Cl-KYN (AV-101)
- 5,7-DCKA
- 7-CKA
- ACC
- ACEA-1011
- ACEA-1328
- AV-101
- Carisoprodol
- CGP-39653
- CNQX
- DNQX
- Felbamate
- Gavestinel
- GV-196,771
- Kynurenic acid
- Kynurenine
- L-689,560
- L-701,324
- Licostinel (ACEA-1021)
- LU-73,068
- MDL-105,519
- Meprobamate
- MRZ 2/576
- PNQX
- ZD-9379; NR2B subunit antagonists: Besonprodil
- CERC-301 (MK-0657)
- CO-101,244 (PD-174,494)
- Eliprodil
- Haloperidol
- Ifenprodil
- Isoxsuprine
- Nylidrin
- Ro8-4304
- Ro25-6981
- Traxoprodil; Polyamine site antagonists: Arcaine
- Co 101676
- Diaminopropane
- Diethylenetriamine
- Huperzine A
- Putrescine
- Ro 25-6981; Unclassified/unsorted antagonists: Bumetanide
- Chloroform
- Cyclopropane
- D-αAA
- Diethyl ether
- Enflurane
- Ethanol
- Flufenamic acid
- Flupirtine
- Furosemide
- Halothane
- Isoflurane
- Metaphit
- Methoxyflurane
- Niflumic acid
- Pentamidine isethionate
- Piretanide
- Toluene
- Transcrocetin (saffron)
- Trichloroethane
- Trichloroethanol
- Trichloroethylene
- Xylene
|
|---|
| | Kainate | |
|---|
| | mGlu1 | |
|---|
| | mGlu2 | |
|---|
| | mGlu3 | |
|---|
| | mGlu4 |
- Antagonists: CPPG
- MAP4
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
| | mGlu5 | |
|---|
| | mGlu6 |
- Antagonists: CPPG
- MAP4
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
| | mGlu7 |
- Antagonists: CPPG
- MAP4
- MMPIP
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
| | mGlu8 |
- Antagonists: CPPG
- MAP4
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
|
|---|
|
Transporter
(blockers) | |
|---|
|
Enzyme
(inhibitors) | |
|---|
|
| Others | |
|---|
|
See also: GABAergics • GHBergics • Glycinergics |
|
|---|
|
| Agonists | |
|---|
|
| Antagonists | |
|---|
|
Unknown /
unsorted | |
|---|