Blonanserin
 | |
| Clinical data | |
|---|---|
| Trade names | Lonasen |
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 55%.[1] |
| Metabolism | CYP3A4[1] |
| Biological half-life | 12 h[1] |
| Excretion | 59% (urine), 30% (faeces)[1] |
| Identifiers | |
| |
| CAS Number | 132810-10-7 |
| PubChem (CID) | 125564 |
| ChemSpider | 111697 |
| UNII |
AQ316B4F8C |
| KEGG | D01176 |
| ChEMBL | CHEMBL178803 |
| Chemical and physical data | |
| Formula | C23H30FN3 |
| Molar mass | 367.50 g/mol |
| 3D model (Jmol) | Interactive image |
| |
Blonanserin (Lonasen) is a relatively new atypical antipsychotic (approved by PMDA in January 2008)[2] commercialized by Dainippon Sumitomo Pharma in Japan and Korea for the treatment of schizophrenia.[3] Relative to many other antipsychotics, blonanserin has an improved tolerability profile, lacking side effects such as extrapyramidal symptoms, excessive sedation, or hypotension.[4] As with many second-generation (atypical) antipsychotics it is significantly more efficacious in the treatment of the negative symptoms of schizophrenia compared to first-generation (typical) antipsychotics such as haloperidol.[5]
Medical Use
The use of atypical antipsychotics as a first-line treatment for schizophrenia is controversial. The National Institute of Mental Health’s CATIE trials suggest that atypical antipsychotics are not significantly more effective against negative symptoms of schizophrenia than typical antipsychotics.[6][7][8][9] Blonanserin contradicts these findings by demonstrating more efficacy in treating negative symptoms than the related typical antipsychotic haloperidol.[10] Despite its suggested advantages, blonanserin does not meet the criteria for Lipinski’s rule, indicating it may not have effective pharmacological or biological activity and predicting that it would not be successful in the clinical trial phases of drug development. Blonanserin is approved for treatment of schizophrenia in Japan and South Korea.[11] Blonanserin is not approved for the same use by the FDA.[11]
Pharmacodynamics
Blonanserin acts as a mixed 5-HT2A (Ki = 0.812 nM) and D2 receptor (Ki = 0.142 nM) antagonist and also exerts some blockade of α1-adrenergic receptors (Ki = 26.7 nM).[12][13] Blonanserin also shows significant affinity for the D3 receptor (Ki = 0.494 nM).[14] It lacks significant affinity for numerous other sites including the 5-HT1A, 5-HT3, D1, α2-adrenergic, β-adrenergic, H1, and mACh receptors and the monoamine transporters,[13] though it does possess low affinity for the sigma receptor (IC50 = 286 nM).[13]
Blonanserin has a relatively high affinity towards the 5-HT6 receptor perhaps underpinning its recently unveiled efficacy in treating the cognitive symptoms of schizophrenia.[12][15] The efficacy of blonanserin can in part be attributed to its chemical structure, which is unique from those of other atypical antipsychotics.[16] Specifically, the addition of hydroxyl groups to blonanserin's unique eight membered ring results in the (R) stereoisomer of the compound demonstrating increased affinity for the indicated targets.[17]
| Receptor | Ki [nM] (Blonanserin)* [12] | Ki [nM] (N-deethylblonanserin)* [3] |
|---|---|---|
| D1 | 1070 | 1020 |
| D2 | 0.142 | 1.38 |
| D3 | 0.494 | 0.23 |
| D4 | 150 | - |
| D5 | 2600 | - |
| 5-HT1A | 804 | - |
| 5-HT2A | 0.812 | 1.28 |
| 5-HT2C | 26.4 | 4.50 |
| 5-HT6 | 11.7 | 5.03 |
| 5-HT7 | 183 | - |
| α1 | 26.7 (Rat brain) | 206 (Rat receptor) |
| α2 | 530 (Rat cloned) | - |
| M1 | 100 | - |
| H1 | 765 | - |
* Towards human receptors unless otherwise specified.
Pharmacokinetics
Blonanserin is administered 4 mg orally 2 times a day or 8 mg once a day, for an adult male with a body mass index between 19–24 kg/m2 and a body weight equal to or greater than 50 kg.[18] The drug is absorbed by a two compartment (central and peripheral) model with first-order absorption and elimination.[19] The half-life of blonanserin is dependent on the dose. A single dose of 4 mg has a half-life of 7.7 ± 4.63 h and a single dose of 8 mg has a half-life of 11.9 ± 4.3 h.[18] The increase of half-life with dose is possibly attributed to there being more individual concentration per time points below the lower limit necessary for quantification in the lower single dose.[18]
Blonanserin is not a charged compound and exhibits very little chemical polarity. The polar surface area of Blonanserin is 19.7 Å[20] It is commonly accepted that a compound needs to have polar surface area less than 90 Å to cross the blood brain barrier so blonanserin is expected to be quite permeable as is demonstrated by a high brain/ plasma ratio of 3.88.[21]
Due to the good permeability of blonanserin, the volume of distribution in the central nervous system is greater than that in the periphery (Vd central = 9500 L, Vd periphery = 8650 L) although it is slower to absorb into the central compartment.[19]
Despite some promising clinical trials[18][19] blonanserin has not met the criteria for Lipinski's rule.[20] A factor contributing to blonanserin’s failure to meet Lipinksi’s rule is that it has a relatively high molecular mass and successful lead compounds are expected to have a molecular mass less than 300 g/mol.
Effect of Feeding
Food intake slows the absorption of blonanserin and increases the bioavailability peripherally relative to centrally.[19] Single fasting doses are safe and the effects of feeding intake are possibly explained by an interaction between blonanserin and Cytochrome P450 3A4 in the gut.[18]
Action at the Dopamine-D3 receptor
The unique indication of blonanserin is antagonistic action at dopamine-D3 receptors (link edit) in the medial prefrontal cortex that potentiates phosphorylation levels of Protein kinase A (PKA) and counteracts decreased activity at the dopamine-D1 and/or NMDA receptors, thus potentiating GABA induced Cl- currents and resulting in improvements in cognitive performance.[14][22] Other atypical antipsychotics, such as olanzapine, fail to show comparable efficacy to rescue PKA activity.[14][23] Many antipsychotics, such as haloperidol, chlorpromazine, risperidone and olanzapine primarily antagonize serotonin 5-HT2A and dopamine-D2 receptors and lack known action at dopamine-D2/3 receptors.[14][16]
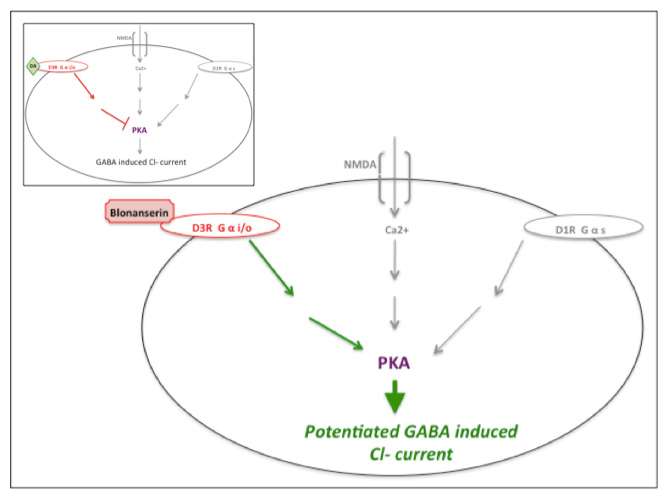 |
| Blonanserin action at dopamine-D3 receptor. Cartoon of blonanserin's antagonistic impact at the dopamine-D3 receptor, reversing inhibition of PKA activity (also regulated by dopamine-D1 and NMDA activity) thus potentiating GABA induced Cl- current. Inset illustrates uninterrupted dopamine (DA) activity at the dopamine-D3 receptor. Inspired by Hida et al. (2014) and Yokota et al. (2002).[14][22] |
Side Effects
As with many of the atypical antipsychotics, blonanserin can elicit cardio metabolic risks. While the side effects of blonanserin – such as weight gain, cholesterol and triglyceride levels, glucose levels and other blood lipid levels – do not differ greatly from other atypical antipsychotics, the specificity of blonanserin appears to elicit milder side effects, with less weight gain in particular.[5]
See also
References
- 1 2 3 4 Wen, YG; Shang, DW; Xie, HZ; Wang, XP; Ni, XJ; Zhang, M; Lu, W; Qiu, C; Liu, X; Li, FF; Li, X; Luo, FT (March 2013). "Population pharmacokinetics of blonanserin in Chinese healthy volunteers and the effect of the food intake". Human Psychopharmacology. 28 (2): 134–141. doi:10.1002/hup.2290. PMID 23417765.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2013-01-19. Retrieved 2013-08-16.
- 1 2 Deeks, ED; Keating, GM (January 2010). "Blonanserin A Review of its Use in the Management of Schizophrenia". CNS Drugs. 24 (1): 65–84. doi:10.2165/11202620-000000000-00000. PMID 20030420.
- ↑ Heading CE (November 1998). "AD-5423 (Dainippon Pharmaceutical Co Ltd)". IDrugs : the Investigational Drugs Journal. 1 (7): 813–7. PMID 18465651.
- 1 2 Kishi, T; Matsuda, Y; Nakamura, H; Iwata, N (Feb 2013). "Blonanserin for schizophrenia: Systematic review and meta-analysis of double-blind, randomized, controlled trials". Journal of Psychiatric Research. 47 (2): 149–54. doi:10.1016/j.jpsychires.2012.10.011.
- ↑ Manschreck, TC; Boshes, RA (2007). "The CATIE schizophrenia trial: results, impact, controversy.". Harvard Review of Psychiatry. 15 (5): 245–58. doi:10.1080/10673220701679838. PMID 17924259.
- ↑ Lieberman, JA (October 2006). "Comparative effectiveness of antipsychotic drugs. A commentary on: Cost Utility Of The Latest Antipsychotic Drugs In Schizophrenia Study (CUtLASS 1) and Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE).". Archives of General Psychiatry. 63 (10): 1069–72. doi:10.1001/archpsyc.63.10.1069. PMID 17015808.
- ↑ Kane, JM (May 2006). "Commentary on the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).". The Journal of Clinical Psychiatry. 67 (5): 831–2. doi:10.4088/jcp.v67n0519. PMID 16841634.
- ↑ McEvoy, JP (July 2006). "An overview of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study.". CNS spectrums. 11 (7 Suppl 7): 4–8. PMID 16816794.
- ↑ Garcia, E; Robert, M; Peris, F; Nakamura, H; Sato, N; Terazawa, Y (2009). "The efficacy and safety of blonanserin compared with haloperidol in acute-phase schizophrenia: a randomized, double-blind, placebo-controlled, multicentre study.". CNS Drugs. 23 (7): 615–25. doi:10.2165/00023210-200923070-00006. PMID 19552488.
- 1 2 Wang, SM; Han, C; Lee, SJ; Patkar, AA; Masand, PS; Pae, CU (2013). "Asenapine, blonanserin, iloperidone, lurasidone, and sertindole: distinctive clinical characteristics of 5 novel atypical antipsychotics.". Clinical neuropharmacology. 36 (6): 223–38. doi:10.1097/wnf.0b013e3182aa38c4. PMID 24201235.
- 1 2 3 Tenjin, T; Miyamoto, S; Ninomiya, Y; Kitajima, R; Ogino, S; Miyake, N; Yamaguchi, N (2013). "Profile of blonanserin for the treatment of schizophrenia". Neuropsychiatric Disease and Treatment. 9: 587–594. doi:10.2147/NDT.S34433. PMC 3677929
 . PMID 23766647.
. PMID 23766647. - 1 2 3 Oka M, Noda Y, Ochi Y, et al. (January 1993). "Pharmacological profile of AD-5423, a novel antipsychotic with both potent dopamine-D2 and serotonin-S2 antagonist properties". The Journal of Pharmacology and Experimental Therapeutics. 264 (1): 158–65. PMID 8093723.
- 1 2 3 4 5 Hida, H; Mouri, A; Mori, K; Matsumoto, Y; Seki, T; Taniguchi, M; Yamada, K; Iwamoto, K; Ozaki, N; Nabeshima, T; Noda, Y (14 August 2014). "Blonanserin Ameliorates Phencyclidine-Induced Visual-Recognition Memory Deficits: the Complex Mechanism of Blonanserin Action Involving D3-5-HT2A and D1-NMDA Receptors in the mPFC.". Neuropsychopharmacology. 40: 601–13. doi:10.1038/npp.2014.207. PMID 25120077.
- ↑ Tenjin, T; Miyamoto, S; Miyake, N; Ogino, S; Kitajima, R; Ojima, K; Arai, J; Teramoto, H; Tsukahara, S; Ito, Y; Tadokoro, M; Anai, K; Funamoto, Y; Kaneda, Y; Sumiyoshi, T; Yamaguchi, N (January 2012). "Effect of blonanserin on cognitive function in antipsychotic-naïve first-episode schizophrenia". Human Psychopharmacology. 27 (1): 90–100. doi:10.1002/hup.1276. PMID 22278973.
- 1 2 Suzuki, K; Hiyama, Y; Une, T; Fujiwara, I (November 2002). "Crystal structure of an antipsychotic agent, 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine (blonanserin).". Analytical Sciences. 18 (11): 1289–90. doi:10.2116/analsci.18.1289. PMID 12458724.
- ↑ Ochi, T; Sakamoto, M; Minamida, A; Suzuki, K; Ueda, T; Une, T; Toda, H; Matsumoto, K; Terauchi, Y (15 February 2005). "Syntheses and properties of the major hydroxy metabolites in humans of blonanserin AD-5423, a novel antipsychotic agent.". Bioorganic & Medicinal Chemistry Letters. 15 (4): 1055–9. doi:10.1016/j.bmcl.2004.12.028. PMID 15686911.
- 1 2 3 4 5 Chen, X; Wang, H; Jiang, J; Chen, R; Zhou, Y; Zhong, W; Liu, H; Hu, P (March 2014). "The pharmacokinetic and safety profiles of blonanserin in healthy Chinese volunteers after single fasting doses and single and multiple postprandial doses.". Clinical drug investigation. 34 (3): 213–22. doi:10.1007/s40261-013-0167-9. PMID 24399453.
- 1 2 3 4 Wen, YG; Shang, DW; Xie, HZ; Wang, XP; Ni, XJ; Zhang, M; Lu, W; Qiu, C; Liu, X; Li, FF; Li, X; Luo, FT (March 2013). "Population pharmacokinetics of blonanserin in Chinese healthy volunteers and the effect of the food intake.". Human psychopharmacology. 28 (2): 134–41. doi:10.1002/hup.2290. PMID 23417765.
- 1 2 "Properties Viewer"..
- ↑ Tateno, A; Arakawa, R; Okumura, M; Fukuta, H; Honjo, K; Ishihara, K; Nakamura, H; Kumita, S; Okubo, Y (Apr 2013). "Striatal and extrastriatal dopamine D2 receptor occupancy by a novel antipsychotic, blonanserin: a PET study with [11C]raclopride and [11C]FLB 457 in schizophrenia.". Journal of Clinical Psychopharmacology. 33 (2): 162–9. doi:10.1097/jcp.0b013e3182825bce. PMID 23422369.
- 1 2 Yokota, K; Tatebayashi, H; Matsuo, T; Shoge, T; Motomura, H; Matsuno, T; Fukuda, A; Tashiro, N (March 2002). "The effects of neuroleptics on the GABA-induced Cl- current in rat dorsal root ganglion neurons: differences between some neuroleptics.". British Journal of Pharmacology. 135 (6): 1547–55. doi:10.1038/sj.bjp.0704608. PMC 1573270
 . PMID 11906969.
. PMID 11906969. - ↑ Nagai, T; Noda, Y; Une, T; Furukawa, K; Furukawa, H; Kan, QM; Nabeshima, T (10 February 2003). "Effect of AD-5423 on animal models of schizophrenia: phencyclidine-induced behavioral changes in mice.". NeuroReport. 14 (2): 269–72. doi:10.1097/00001756-200302100-00023. PMID 12598744.