Muscarine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,5-Anhydro-1,4,6-trideoxy-6-(trimethylammonio)-D-ribo-hexitol | |
| Other names
L-(+)-muscarine, muscarin, (2S,4R,5S)-(4-hydroxy-5-methyl-tetrahydrofuran-2-ylmethyl)-trimethyl-ammonium | |
| Identifiers | |
| 300-54-9 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL12587 |
| ChemSpider | 8949 |
| ECHA InfoCard | 100.005.541 |
| 3996 | |
| PubChem | 9308 |
| |
| |
| Properties | |
| C9H20NO2+ | |
| Molar mass | 174.26 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |

Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Muscarine is only a trace compound in the fly agaric Amanita muscaria; the pharmacologically more relevant compound from this mushroom is muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.[1]
Muscarine is a nonselective agonist of the muscarinic acetylcholine receptor.
History
Muscarine was first isolated from Amanita muscaria by German chemists Oswald Schmiedeberg and Richard Koppe, who reported their findings in 1869.[2] It was the first parasympathomimetic substance ever studied and causes profound activation of the peripheral parasympathetic nervous system that may end in convulsions and death. Being a quaternary ammonium salt, muscarine is less completely absorbed from the gastrointestinal tract than tertiary amines, but it does cross the blood-brain barrier.[3] Muscarinic agonists activate muscarinic receptors while nicotinic agonists activate nicotine receptors. Both are direct-acting cholinomimetics; they produce their effects by binding to and activating cholinergic receptors. Final proof of the structure was given by Franz Jellinek and colleagues in 1957 with the help of X-ray diffraction analysis;[4] Jellinek further described the three-dimensional structure of the molecule using muscarine chloride.[5] These new findings set into motion research not only on the pharmacology of muscarine, but also on that of muscarine-like substances that are structurally related to acetylcholine.
Structure and reactivity
‘Muscarine mimics the function of the natural neurotransmitter acetylcholine in the muscarinic part of the cholinergic nervous system", despite the less flexible structure due to the five-membered ring in the molecular skeleton.[6]
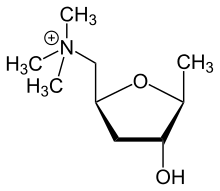
There are two mirror forms of muscarine, named: 2S-muscarine and 2R-muscarine.
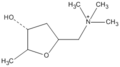 Figure 1. The structural formula of 2S-muscarine.
Figure 1. The structural formula of 2S-muscarine. Figure 2. The structural formula of 2R-muscarine.
Figure 2. The structural formula of 2R-muscarine.
Efficient synthesis of (+)-muscarine
The next is a very efficient way of synthesis of (+)-muscarine according to the scientists Chan and Li in the Canadian journal of Chemistry in 1992.[7] S-(−)-Ethyl lactate (2)(figure 3) is converted into the 2,6-dichlorobenzyl ether (3). Diisobutylaluminium hydride (DIBAL) reduction of the 2,6-dichlorobenzyl ether gives the aldehyde (4). Treatment of the crude aldehyde with allyl bromide and zinc powder in water with NH4Cl as catalyst resulted in an anti:syn mixture of 5a and 5b. Treatment of 5a with iodine in CH3CN at 0 °C gives the cyclized product 6a. Finally treatment of 6a with excess trimethylamine in ethanol gave (+)-muscarine (2S,4R,5S). A similar reaction sequence with 5b gave (+)-epimuscarine (7).[7]
-muscarin.gif)
Other Syntheses
It can be synthesized in various ways from completely different substances,[8][9][10][11][12][13][14][15][16][17] particularly from 2,5-dimethyl-3-carboxymethyl flurane.
Mechanism of action
Muscarine mimics the action of the neurotransmitter acetylcholine by binding muscarinic acetylcholine receptors. These receptors were named after muscarine. There are 5 different types of muscarinic receptors; M1 - M5, and most tissues express a mixture of subtypes. The M2 and M3 subtypes mediate muscarinic responses at peripheral autonomic tissues. M1 and M4 subtypes are more abundant in brain and autonomic ganglia. M1, M3 and M5 interact with Gq proteins to stimulate phosphoinositide hydrolysis and the release of intracellular calcium. M2 and M4 receptors interact with Gi proteins to inhibit adenylyl cyclase, which results in a decrease of intracellular concentration of cyclic adenosine monophosphate (cAMP). Most agonists for muscarine receptors are not selective for subtypes.[18]
Metabolism
A paucity of research exists on the metabolism of muscarine in the human body, suggesting this compound is not metabolized by humans. Though there has been extensive research in the field of acetylcholine metabolism by acetylcholinesterase, muscarine is not metabolized by this enzyme, partly explaining the compound's potential toxicity. Muscarine is readily soluble in water. The most likely way for muscarine to leave the blood is via renal clearance; it will eventually leave the body in urine.[19]
Drug
Muscarinic agonists are used as drugs in treating glaucoma, postoperative ileus, congenital megacolon, urinary retention and xerostomia. Muscarine is contraindicated in patients with diseases that make them susceptible to parasympathetic stimulation, patients who have asthma or COPD or patients who have peptic ulcer disease. Also patients with an obstruction in the gastrointestinal or urinary tract are not prescribed muscarine because it will aggravate the obstruction, causing pressure to build up that may lead to perforation.
Efficacy
As muscarine works on the muscarinic acetylcholine receptor the best comparison can be made with acetylcholine, which normally works on this receptor. Pure muscarine compared to pure acetylcholine is stated in most cases to be more potent, its action is always slower but longer lasting than acetylcholine. A possible explanation for this long lasting behavior might be that muscarine does not get hydrolyzed by acetylcholinesterase in the synaptic cleft.[20]
Toxicology
Muscarine poisoning is characterized by miosis, blurred vision, increased salivation, excessive sweating, lacrimation, bronchial secretions, bronchoconstriction, bradycardia, abdominal cramping, increased gastic acid secretion, diarrhea and polyuria. If muscarine reaches the brain it can cause tremor, convulsions and hypothermia. Cardiac ventricles contain muscarinic receptors that mediate a decrease in the force of contractions leading to a lower blood pressure. If muscarine is administered intravenously, muscarine can trigger acute circulatory failure with cardiac arrest.[1] The symptoms of intoxication with mushrooms rich in muscarine, especially Inocybe, are very typical: The symptoms start early, after one-quarter to two hours, with headache, nausea, vomiting, and constriction of the pharynx. Then salivation, lacrimation, and diffuse perspiration set in, combined with miosis, disturbed accommodation, and reduced vision. Gastric and small bowel colic leads to diarrhea, and there is a painful urge for urination. Bronchoconstriction leads to asthmatic attacks and severe dyspnea, and bradycardia combined with marked hypotension and vasodilation results in circulatory shock. Death after 8 to 9 hours has been reported in about 5% of the cases, but can be avoided completely by prompt diagnosis and treatment with atropine.[21]
Antidote
The specific antidote is atropine. Atropine is also an alkaloid and inhibits acetylcholine and thus muscarine by binding to muscarinic receptors. Other muscarinic antagonists are scopolamine and pirenzepine. Muscarinic antagonists dilate the pupil and relax the ciliary muscle, are used in treatment of inflammatory uveitis and is associated with glaucoma. They are also used to treat urinary incontinence and diseases characterized by bowel hypermotility such as irritable bowel syndrome. Muscarinic antagonists are often called parasympatholytics because they have the same effect as agents that block postganglionic parasympatic nerves.
References
- 1 2 Lurie, Y; Wasser, SP; Taha, M; Shehade, H; Nijim, J; Hoffmann, Y; Basis, F; Vardi, M; Lavon, O; Suaed, S; Bisharat, B; Bentur, Y (July 2009). "Mushroom poisoning from species of genus Inocybe (fiber head mushroom): a case series with exact species identification". Clinical toxicology (Philadelphia, Pa.). 47 (6): 562–5. doi:10.1080/15563650903008448. PMID 19566380.
- ↑ Schmiedeberg, O.; Koppe, R. (1869). Das Muscarin, das giftige Alkaloid des Fliegenpilzes (Agaricus muscarius L.), seine Darstellung, chemischen Eigenschaften, physiologischen Wirkungen, toxicologische Bedeutung und sein Verhältniss zur Pilzvergiftung im allgemeinen [Muscarine, the poisonous alkaloid of the fly agaric (Agaricus muscarius L.), its preparation, chemical properties, physiological effects, toxicological importance, and its relation to mushroom poisoning in general]. Leipzig: Verlag von F.C.W. Vogel.
- ↑ Pappano Achilles J, "Chapter 7. Cholinoceptor-Activating & Cholinesterase-Inhibiting Drugs" (Chapter). Katzung BG: Basic & Clinical Pharmacology, 11e
- ↑ Kögl, F.; Salemink, C.A.; Schouten, H.; Jellinek, F. (1957). "Über Muscarin. III". Recueil des Travaux Chimiques des Pays-Bas (in German). 76 (2): 109–127. doi:10.1002/recl.19570760204.
- ↑ Jellinek, F. (1957). "The structure of muscarine". Acta Crystallographica. 10: 277–280. doi:10.1107/S0365110X57000845.
- ↑ Frydenvang, K.; Jensen, B. (15 May 1993). "Structures of muscarine picrate and muscarine tetraphenylborate". Acta Crystallographica Section C. 49 (5): 985–990. doi:10.1107/S0108270192012198.
- 1 2 Chan, T. H.; Li, C. J. (November 1992). "A concise synthesis of (+)-muscarine". Canadian Journal of Chemistry. 70 (11): 2726–2729. doi:10.1139/v92-346.
- ↑ Kögl, F.; Salemink, C. A.; Schouten, H.; Jellinek, F. (2010). "Über Muscarin. III". Recueil des Travaux Chimiques des Pays-Bas. 76 (2): 109. doi:10.1002/recl.19570760204.
- ↑ Kögl, F.; Cox, H. C.; Salemink, C. A. (1957). "Über Muscarin". Experientia. 13 (4): 137. doi:10.1007/BF02158130.
- ↑ Corrodi, H.; Hardegger, E.; Kögl, F.; Zeller, P. (1957). "Synthese von Stereoisomeren des Muscarins". Experientia. 13 (4): 138. doi:10.1007/BF02158131.
- ↑ Cox, H. C.; Hardegger, E.; Kögl, F.; Liechti, P.; Lohse, F.; Salemink, C. A. (1958). "Über Muscarin. 9. Mitteilung. Über die Synthese von racemischem Muscarin, seine Spaltung in die Antipoden und die Herstellung von (−)-Muscarin aus D-Glucosamin". Helvetica Chimica Acta. 41: 229. doi:10.1002/hlca.660410129.
- ↑ Matsumoto, T.; Ichihara, A.; Ito, N. (1969). "A simple, stereospecific synthesis of dl-muscarine and dl-allomuscarine". Tetrahedron. 25 (24): 5889. doi:10.1016/S0040-4020(01)83096-9.
- ↑ Still, W. C.; Schneider, J. A. (1980). "Chelation-controlled synthesis of (.+-.)-muscarine". The Journal of Organic Chemistry. 45 (16): 3375. doi:10.1021/jo01304a056.
- ↑ Whiting, J.; Au-Young, Y. -K.; Belleau, B. (1972). "A Convenient Synthesis ofL(+)-Muscarine". Canadian Journal of Chemistry. 50 (20): 3322. doi:10.1139/v72-532.
- ↑ Mubarak, A. M.; Brown, D. M. (1980). "A simple, stereospecific synthesis of (+)-muscarine". Tetrahedron Letters. 21 (25): 2453. doi:10.1016/S0040-4039(00)93174-5.
- ↑ Mubarak, A. M.; Brown, D. M. (1982). "A stereospecific synthesis of (+)-muscarine". Journal of the Chemical Society, Perkin Transactions 1: 809. doi:10.1039/P19820000809.
- ↑ Pochet, S.; Huynh Dinh Tam (1982). "Stereospecific synthesis of muscarines and allomuscarines in D- and L-series". The Journal of Organic Chemistry. 47 (2): 193. doi:10.1021/jo00341a003.
- ↑ Theodore M. Brody; Joseph Larner; Kenneth P. Minneman, eds. (1998). "Chapter 9". Human Pharmacology: Molecular to Clinical (3rd ed.). St. Louis, Mo.: Mosby. ISBN 0815124562.
- ↑ Roberts Bartholow, “A practical treatise on materia medica and therapeutics”, 1908, ISBN 978-1-143-46767-7,
- ↑ Fraser, PJ (March 1957). "Pharmacological actions of pure muscarine chloride". Br J Pharmacol Chemother. 12 (1): 47–52. doi:10.1111/j.1476-5381.1957.tb01361.x. PMC 1509643
 . PMID 13413151.
. PMID 13413151. - ↑ Peter G. Waser; Chemistry and pharmacology of muscarine, muscarone and some related compounds; Pharmacology Department, University of Zurich, Switzerland 1961
