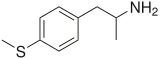
4-Methylthioamphetamine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
14116-06-4 1030831-15-2 (R) 943816-61-3 (S) |
| PubChem (CID) | 151900 |
| ChemSpider |
133883 |
| ChEMBL |
CHEMBL6467 |
| Chemical and physical data | |
| Formula | C10H15NS |
| Molar mass | 181.299 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
4-Methylthioamphetamine (4-MTA) is a designer drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic highly selective serotonin releasing agent (SSRA) in animals.[1][2][3] It is distantly related to several other SSRAs, including MMAI, MDAI, and MDMAI.
Effects
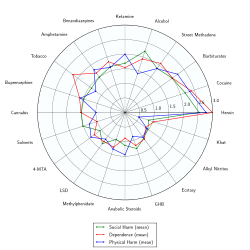
4-MTA is a strong serotonin releaser similar to paramethoxyamphetamine (PMA), which can cause pronounced hyperthermia potentially resulting in organ failure and death.[5][6][7][8] The subjective effects of 4-MTA include prolonged stimulation, which, in contrast to other amphetamines, is accompanied by little sense of euphoria.[9] 4-MTA is also an MAO-A inhibitor, which may explain its tendency to easily cause adverse effects, as MAOIs are not considered safe to use in conjunction with stimulants.[10][11][12]
History
It was developed by the research team led by David E. Nichols,[13] but was intended to be used only as an agent for laboratory research into the serotonin transporter protein, and Nichols was reportedly sad to see 4-MTA appear as a drug of abuse on the street.[14]
4-MTA was briefly sold in smart shops in the Netherlands, though was soon banned by the Dutch government after serious side-effects started to emerge. It was also briefly sold on the black market as MDMA during the late 1990s, mainly in the USA, but proved unpopular due to its high risk of severe side effects (several deaths were reported) and relative lack of positive euphoria.
Legality
United Kingdom
4-MTA is currently a Class A drug in the United Kingdom although it has been suggested it be rescheduled as a Class B drug.[15] It is also a controlled substance in Argentina (as well as 2C-B, 2C-I and 2C-T-2).[16]
United States
4-MTA is currently unregulated at the federal level; however, it is listed as a controlled substance under Wisconsin law.[17]
See also
References
- ↑ Huang X, Marona-Lewicka D, Nichols DE (1992). "p-methylthioamphetamine is a potent new non-neurotoxic serotonin-releasing agent.". Eur J Pharmacol. 229 (1): 31–38. doi:10.1016/0014-2999(92)90282-9. PMID 1473561.
- ↑ Li, Q; Murakami, I; Stall, S; Levy, AD; Brownfield, MS; Nichols, DE; Van De Kar, LD (1996). "Neuroendocrine pharmacology of three serotonin releasers: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi) and p-methylthioamphetamine (MTA).". The Journal of Pharmacology and Experimental Therapeutics. 279 (3): 1261–7. PMID 8968349.
- ↑ Murphy, J; Flynn, JJ; Cannon, DM; Guiry, PJ; McCormack, P; Baird, AW; McBean, GJ; Keenan, AK (2002). "In vitro neuronal and vascular responses to 5-hydroxytryptamine: modulation by 4-methylthioamphetamine, 4-methylthiomethamphetamine and 3,4-methylenedioxymethamphetamine.". European Journal of Pharmacology. 444 (1–2): 61–7. doi:10.1016/S0014-2999(02)01586-8. PMID 12191583.
- ↑ Nutt, D; King, LA; Saulsbury, W; Blakemore, C (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse.". Lancet (London, England). 369 (9566): 1047–53. PMID 17382831.
- ↑ Elliott, SP (2000). "Fatal poisoning with a new phenylethylamine: 4-methylthioamphetamine (4-MTA)". Journal of analytical toxicology. 24 (2): 85–89. doi:10.1093/jat/24.2.85. PMID 10732944.
- ↑ De Letter, EA; Coopman, VA; Cordonnier, JA; Piette, MH (2001). "One fatal and seven non-fatal cases of 4-methylthioamphetamine (4-MTA) intoxication: clinico-pathological findings". International journal of legal medicine. 114 (6): 352–6. doi:10.1007/s004140100204. PMID 11508803.
- ↑ Decaestecker, T; De Letter, E; Clauwaert, K; Bouche, MP; Lambert, W; Van Bocxlaer, J; Piette, M; Van Den Eeckhout, E; Van Peteghem, C; De Leenheer, A (2001). "Fatal 4-MTA intoxication: development of a liquid chromatographic-tandem mass spectrometric assay for multiple matrices". Journal of analytical toxicology. 25 (8): 705–10. doi:10.1093/jat/25.8.705. PMID 11765028.
- ↑ Smets, G; Bronselaer, K; De Munnynck, K; De Feyter, K; Van De Voorde, W; Sabbe, M (2005). "Amphetamine toxicity in the emergency department". European Journal of Emergency Medicine. 12 (4): 193–7. doi:10.1097/00063110-200508000-00010. PMID 16034267.
- ↑ Erowid experience report on 4-MTA (experience year: 2001)
- ↑ Carmo, H; Remião, F; Carvalho, F; Fernandes, E; De Boer, D; Dos Reys, LA; De Lourdes Bastos, M (2003). "4-Methylthioamphetamine-induced hyperthermia in mice: influence of serotonergic and catecholaminergic pathways". Toxicology and applied pharmacology. 190 (3): 262–71. doi:10.1016/S0041-008X(03)00190-X. PMID 12902197.
- ↑ Hurtado-Guzmán, C; Fierro, A; Iturriaga-Vásquez, P; Sepúlveda-Boza, S; Cassels, BK; Reyes-Parada, M (2003). "Monoamine oxidase inhibitory properties of optical isomers and N-substituted derivatives of 4-methylthioamphetamine". Journal of enzyme inhibition and medicinal chemistry. 18 (4): 339–47. doi:10.1080/1475636031000118437. PMID 14567549.
- ↑ Carmo, H.; Brulport, M.; Hermes, M.; Oesch, F.; De Boer, D.; Remião, F.; Carvalho, F. L.; Schön, M. R.; Krebsfaenger, N.; Doehmer, J.; Bastos Mde, M. D. L.; Hengstler, J. G. (2007). "CYP2D6 increases toxicity of the designer drug 4-methylthioamphetamine (4-MTA)". Toxicology. 229 (3): 236–244. doi:10.1016/j.tox.2006.10.024. PMID 17156908.
- ↑ Huang, X; Marona-Lewicka, D; Nichols, DE (1992). "p-methylthioamphetamine is a potent new non-neurotoxic serotonin-releasing agent". European Journal of Pharmacology. 229 (1): 31–38. doi:10.1016/0014-2999(92)90282-9. PMID 1473561.
- ↑ 4-MTA info on possible dangers - ecstasy.org
- ↑ ""I think 4MTA, LSD and ecstasy probably shouldn't be Class A", from "Call for ecstasy to be downgraded", BBC News, Wednesday, 22 November 2006, 15:57 GMT
- ↑ Last Argentina Controlled Drugs List
- ↑ "4-MTA Legal Status". Erowid. June 21, 2004. Retrieved 27 March 2014.
External links
- EMCDDA Report (1999) on the risk assessment of 4-MTA in the framework of the joint action on new synthetic drugs
- Erowid 4-MTA vault (Accessed 10/1/06)
- Ecstasy.org: 4-MTA - info on possible dangers (Accessed 10/1/06)
- Abstract: "Para-methylthioamphetamine, A New Designer Drug of Abuse" (Accessed 10/1/06)