Arginine
 | |
 | |
| Names | |
|---|---|
| Other names
2-Amino-5-guanidinopentanoic acid | |
| Identifiers | |
| 7200-25-1 157-06-2 R 74-79-3 S | |
| 3D model (Jmol) | Interactive image Interactive image |
| 3DMet | B01331 |
| 1725411, 1725412 R, 1725413 S | |
| ChEBI | CHEBI:29016 |
| ChEMBL | ChEMBL212301 ChEMBL1485 |
| ChemSpider | 227 64224 R 6082 S |
| DrugBank | DB00125 |
| ECHA InfoCard | 100.000.738 |
| EC Number | 230-571-3 |
| 364938 R | |
| 721 | |
| KEGG | C02385 |
| MeSH | Arginine |
| PubChem | 232 71070 R 6322 S |
| RTECS number | CF1934200 S |
| UNII | 94ZLA3W45F |
| |
| |
| Properties | |
| C6H14N4O2 | |
| Molar mass | 174.20 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Melting point | 260 °C; 500 °F; 533 K |
| Boiling point | 368 °C (694 °F; 641 K) |
| 14.87 g/100 mL (20 °C) | |
| Solubility | slightly soluble in ethanol insoluble in ethyl ether |
| log P | −1.652 |
| Acidity (pKa) | 12.488 |
| Basicity (pKb) | 1.509 |
| Thermochemistry | |
| 232.8 J K−1 mol−1 (at 23.7 °C) | |
| Std molar entropy (S |
250.6 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
−624.9–−622.3 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−3.7396–−3.7370 MJ mol−1 |
| Pharmacology | |
| B05XB01 (WHO) S | |
| Hazards | |
| Safety data sheet | See: data page sigma-aldrich |
| GHS pictograms |  |
| GHS signal word | WARNING |
| H319 | |
| P305+351+338 | |
| EU classification (DSD) |
|
| R-phrases | R36 |
| S-phrases | S26 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
5110 mg/kg (rat, oral) |
| Related compounds | |
| Related alkanoic acids |
|
| Related compounds |
|
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
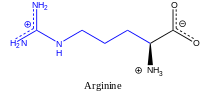
Arginine (abbreviated as Arg or R) encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG[1] is an α-amino acid that is used in the biosynthesis of proteins.
Arginine is classified as a semiessential or conditionally essential amino acid, depending on the developmental stage and health status of the individual.[2] Preterm infants are unable to synthesize or create arginine internally, making the amino acid nutritionally essential for them.[3] Most healthy people do not need to supplement with arginine because their body produces sufficient amounts.[4]
Arginine was first isolated from a lupin seedling extract in 1886 by the German chemist Ernst Schultze.[5] It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain of a 3-carbon aliphatic straight chain capped by a complex guanidinium, classifying it as a charged (at physiological pH), aliphatic amino acid.
Sources
Dietary sources
A conditionally essential amino acid is one that may be required depending on the health status or life cycle of the individual. Arginine is one such conditionally essential amino acid. The biosynthetic pathway, however, does not produce sufficient arginine, and some must still be consumed through diet. Individuals with poor nutrition or certain physical conditions may be advised to increase their intake of foods containing arginine. Arginine is found in a wide variety of foods, including:
- Animal sources
- dairy products (e.g., cottage cheese, ricotta, milk, yogurt, whey protein drinks), beef, pork (e.g., bacon, ham), gelatin, poultry (e.g. chicken and turkey light meat), wild game (e.g. pheasant, quail), seafood (e.g., halibut, lobster, salmon, shrimp, snails, tuna)
- Plant sources
- wheat germ and flour, lupins, buckwheat, granola, oatmeal, peanuts, nuts (coconut, pecans, cashews, walnuts, almonds, Brazil nuts, hazelnuts, pinenuts), seeds (hemp, pumpkin, sesame, sunflower), chickpeas, cooked soybeans, Phalaris canariensis (canaryseed or alpiste)
Biosynthesis
Arginine is synthesized from citrulline in arginine and proline metabolism by the sequential action of the cytosolic enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). In terms of energy, this is costly, as the synthesis of each molecule of argininosuccinate requires hydrolysis of adenosine triphosphate (ATP) to adenosine monophosphate (AMP), i.e., two ATP equivalents. In essence, taking an excess of arginine gives more energy by saving ATPs that can be used elsewhere.
Citrulline can be derived from multiple sources:
- from arginine via nitric oxide synthase (NOS)
- from ornithine via catabolism of proline or glutamine/glutamate
- from asymmetric dimethylarginine (ADMA) via DDAH
The pathways linking arginine, glutamine, and proline are bidirectional. Thus, the net utilization or production of these amino acids is highly dependent on cell type and developmental stage.
On a whole-body basis, synthesis of arginine occurs principally via the intestinal–renal axis, wherein epithelial cells of the small intestine, which produce citrulline primarily from glutamine and glutamate, collaborate with the proximal tubule cells of the kidney, which extract citrulline from the circulation and convert it to arginine, which is returned to the circulation. As a consequence, impairment of small bowel or renal function can reduce endogenous arginine synthesis, thereby increasing the dietary requirement.
Synthesis of arginine from citrulline also occurs at a low level in many other cells, and cellular capacity for arginine synthesis can be markedly increased under circumstances that also induce iNOS. Thus, citrulline, a coproduct of the NOS-catalyzed reaction, can be recycled to arginine in a pathway known as the citrulline-NO or arginine-citrulline pathway. This is demonstrated by the fact that, in many cell types, citrulline can substitute for arginine to some degree in supporting NO synthesis. However, recycling is not quantitative because citrulline accumulates along with nitrate and nitrite, the stable end-products of NO, in NO-producing cells.[6]
Function
Arginine plays an important role in cell division, the healing of wounds, removing ammonia from the body, immune function, and the release of hormones.[2][7][8]
The roles of arginine include:
- Precursor for the synthesis of nitric oxide (NO)[9] Non-L-arginine derived NO can be generated by the nitrate-nitrite-nitric oxide pathway that is monitored through saliva testing.
- Reduces healing time of injuries (particularly bone)[7][8]
- Quickens repair time of damaged tissue[7][8]
- Helps decrease blood pressure in clinical hypertensive subjects[10][11][12] NO-mediated decrease in blood pressure is influenced by both the L-arginine-dependent nitric oxide synthase pathway and non-L-arginine or alternative pathway through nitrate-rich foods such as beets and spinach.
- Arginine is a potent agonist of the mTOR protein kinase that regulates growth and metabolism at both the cellular and organismal level.[13][14] Arginine helps to activate mTORC1 by promoting its localization to the lysosome by binding to the CASTOR proteins.[15][16]
Proteins
The distributing basics of the moderate structure found in geometry, charge distribution, and ability to form multiple H-bonds make arginine ideal for binding negatively charged groups. For this reason, arginine prefers to be on the outside of the proteins, where it can interact with the polar environment.
Incorporated in proteins, arginine can also be converted to citrulline by PAD enzymes. In addition, arginine can be methylated by protein methyltransferases.
Precursor
Arginine is the immediate precursor of nitric oxide (NO), urea, ornithine, and agmatine; is necessary for the synthesis of creatine; and can also be used for the synthesis of polyamines (mainly through ornithine and to a lesser degree through agmatine), citrulline, and glutamate. As a precursor of nitric oxide, arginine may have a role in the treatment of some conditions where vasodilation is required.[2] The presence of asymmetric dimethylarginine (ADMA), a close relative, inhibits the nitric oxide reaction; therefore, ADMA is considered a marker for vascular disease, just as L-arginine is considered a sign of a healthy endothelium.
Treatment of dentin hypersensitivity
Arginine (8%) in dental products (e.g., toothpaste) provides effective relief from sensitive teeth by depositing a dentin-like mineral, containing calcium and phosphate, within the dentin tubules and in a protective layer on the dentin surface.[17]
Treatment of herpes simplex virus
An unproven claim is that a low ratio of arginine to lysine may be of benefit in the treatment of herpes simplex virus. For more information, refer to Herpes – Treatment also see journal article.[18]
Treatment of peripheral neuropathy
A number of studies have shown that L-arginine can have a positive effect in reducing the pain associated with peripheral neuropathy. As the immediate precursor of Nitric Oxide, increased L-arginine intake sets off a cascade of bio-chemical events that ultimately leads to increased blood perfusion in the areas affected by the disease. As more nutrient-rich, oxygenated blood becomes available to the damaged nerve cells, inflammation is reduced and the cells can begin to regenerate. Most studies show L-arginine efficacy in treating peripheral neuropathy is best accomplished with a daily intake of 500 mg to 1000 mg.
Safety
L-arginine is generally recognized as safe (GRAS-status) at intakes of up to 20 g/d.[19]
Structure
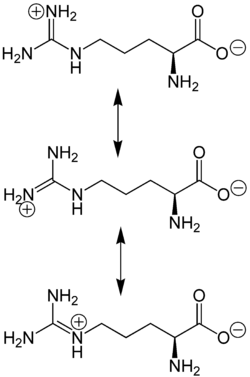
The amino acid side-chain of arginine consists of a 3-carbon aliphatic straight chain, the distal end of which is capped by a complex guanidinium group.
With a pKa of 12.48, the guanidinium group is positively charged in neutral, acidic, and even most basic environments, and thus imparts basic chemical properties to arginine. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized, enabling the formation of multiple H-bonds.
Research
Growth hormone
Intravenously-administered arginine stimulates the secretion of growth hormone,[20] and is used in growth hormone stimulation tests.[21] Two studies have found that oral arginine supplementation is also effective at increasing resting GH levels. The first study found that oral preparations of arginine are effective at increasing growth hormone levels. In fact, the 9-gram dose resulted in mean peak GH levels of 6.4 (± 1.3) µg/L versus placebo levels of 2.9 (± 0.7).[22] Another study found similar results. It included resting versus exercise and oral L-arginine versus oral placebo. The authors concluded that "Oral arginine alone (7 g) stimulated GH release, but a greater GH response was seen with exercise alone. The combined effect of arginine before exercise attenuates the GH response… GH production: Ex > Arg+Ex > Arg > placebo" suggesting against supplementing with arginine alone prior to exercise if the goal is to raise GH levels, but concurring with the previous study that oral L-arginine increases GH on days free of significant exercise.[23] In contrast to these two studies that found increased resting GH due to oral arginine supplementation, a third study did not find increase in resting GH levels from oral supplementation. In that study, oral preparations of L-arginine were ineffective at increasing growth hormone levels despite being effective at increasing plasma levels of L-arginine.[24]
MELAS syndrome
Several trials delved into effects of L-arginine in MELAS syndrome, a mitochondrial disease.[25]
High blood pressure
Intravenous infusion of arginine reduces blood pressure in patients with hypertension as well as normal subjects.[26]
A meta-analysis showed that L-arginine reduces blood pressure with pooled estimates of 5.4/2.7 mmHg for SBP/DBP.[12]
Supplementation with L-arginine reduces diastolic blood pressure and lengthens pregnancy for women with gestational hypertension, including women with high blood pressure as part of pre-eclampsia.[27] It does not lower systolic blood pressure or improve the baby's weight at birth.
See also
- Arginine glutamate
- AAKG
- Canavanine and canaline are toxic analogs of arginine and ornithine.
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Archived from the original on 29 May 2007. Retrieved 2007-05-17.
- 1 2 3 Tapiero H, Mathé G, Couvreur P, Tew KD (November 2002). "L-Arginine". (review). Biomedicine & Pharmacotherapy. 56 (9): 439–445. doi:10.1016/s0753-3322(02)00284-6.
- ↑ Wu G, Jaeger LA, Bazer FW, Rhoads JM (Aug 2004). "Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications". (review). The Journal of Nutritional Biochemistry. 15 (8): 442–51. doi:10.1016/j.jnutbio.2003.11.010. PMID 15302078.
- ↑ "Drugs and Supplements Arginine". http://www.mayoclinic.org/. Retrieved 15 January 2015. External link in
|website=(help) - ↑ Saini, Rashmi; Badole, Sachin L.; Zanwar, Anand A. (2013). "Arginine Derived Nitric Oxide: Key to Healthy Skin". In Watson, Ronald Ross; Zibadi, Sherma. Bioactive Dietary Factors and Plant Extracts in Dermatology. Nutrition and Health. pp. 73–82. doi:10.1007/978-1-62703-167-7_8. ISBN 978-1-62703-166-0.
- ↑ Morris SM (Oct 2004). "Enzymes of arginine metabolism". (review). The Journal of Nutrition. 134 (10 Suppl): 2743S–2747S; discussion 2765S–2767S. PMID 15465778.
- 1 2 3 Stechmiller JK, Childress B, Cowan L (Feb 2005). "Arginine supplementation and wound healing". (review). Nutrition in Clinical Practice. 20 (1): 52–61. doi:10.1177/011542650502000152. PMID 16207646.
- 1 2 3 Witte MB, Barbul A (2003). "Arginine physiology and its implication for wound healing". (review). Wound Repair and Regeneration. 11 (6): 419–23. doi:10.1046/j.1524-475X.2003.11605.x. PMID 14617280.
- ↑ Andrew PJ, Mayer B (Aug 1999). "Enzymatic function of nitric oxide synthases". (review). Cardiovascular Research. 43 (3): 521–31. doi:10.1016/S0008-6363(99)00115-7. PMID 10690324.
- ↑ Gokce N (Oct 2004). "L-arginine and hypertension". (review). The Journal of Nutrition. 134 (10 Suppl): 2807S–2811S; discussion 2818S–2819S. PMID 15465790.
- ↑ Rajapakse NW, De Miguel C, Das S, Mattson DL (Dec 2008). "Exogenous L-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats". (primary). Hypertension. 52 (6): 1084–90. doi:10.1161/HYPERTENSIONAHA.108.114298. PMC 2680209
 . PMID 18981330.
. PMID 18981330. - 1 2 Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W (Dec 2011). "Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials". review. American Heart Journal. 162 (6): 959–965. doi:10.1016/j.ahj.2011.09.012. PMID 22137067.
- ↑ Goberdhan, Deborah C. I.; Wilson, Clive; Harris, Adrian L. (2016-04-12). "Amino Acid Sensing by mTORC1: Intracellular Transporters Mark the Spot". Cell Metabolism. 23 (4): 580–589. doi:10.1016/j.cmet.2016.03.013. ISSN 1932-7420. PMID 27076075.
- ↑ Kennedy, Brian K.; Lamming, Dudley W. (2016-06-14). "The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging". Cell Metabolism. 23 (6): 990–1003. doi:10.1016/j.cmet.2016.05.009. ISSN 1932-7420. PMC 4910876
 . PMID 27304501.
. PMID 27304501. - ↑ Chantranupong, Lynne; Scaria, Sonia M.; Saxton, Robert A.; Gygi, Melanie P.; Shen, Kuang; Wyant, Gregory A.; Wang, Tim; Harper, J. Wade; Gygi, Steven P. (2016-03-24). "The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway". Cell. 165 (1): 153–164. doi:10.1016/j.cell.2016.02.035. ISSN 1097-4172. PMC 4808398
 . PMID 26972053.
. PMID 26972053. - ↑ Saxton, Robert A.; Chantranupong, Lynne; Knockenhauer, Kevin E.; Schwartz, Thomas U.; Sabatini, David M. (2016-08-03). "Mechanism of arginine sensing by CASTOR1 upstream of mTORC1". Nature. 536 (7615): 229–233. doi:10.1038/nature19079. ISSN 1476-4687. PMID 27487210.
- ↑ Petrou I, Heu R, Stranick M, Lavender S, Zaidel L, Cummins D, Sullivan RJ, Hsueh C, Gimzewski JK (2009). "A breakthrough therapy for dentin hypersensitivity: how dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth". (primary). J Clin Dent. 20 (1): 23–31. PMID 19489189.
- ↑ Naito T, Irie H, Tsujimoto K, Ikeda K, Arakawa T, Koyama AH (Apr 2009). "Antiviral effect of arginine against herpes simplex virus type 1". (review). International Journal of Molecular Medicine. 23 (4): 495–499. doi:10.3892/ijmm_00000156. PMID 19288025.
- ↑ Shao A, Hathcock JN (2008). "Risk assessment for the amino acids taurine, L-glutamine and L-arginine". Regul Toxicol Pharmacol. 50 (3): 376–399. doi:10.1016/j.yrtph.2008.01.004.
- ↑ Alba-Roth J, Müller OA, Schopohl J, von Werder K (Dec 1988). "Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion". The Journal of Clinical Endocrinology and Metabolism. 67 (6): 1186–9. doi:10.1210/jcem-67-6-1186. PMID 2903866.
- ↑ U.S. National Library of Medicine (September 2009 Growth hormone stimulation test
- ↑ Collier SR, Casey DP, Kanaley JA (Apr 2005). "Growth hormone responses to varying doses of oral arginine". (primary). Growth Hormone & IGF Research. 15 (2): 136–139. doi:10.1016/j.ghir.2004.12.004. PMID 15809017.
- ↑ Collier SR, Collins E, Kanaley JA (Sep 2006). "Oral arginine attenuates the growth hormone response to resistance exercise". (primary). Journal of Applied Physiology. 101 (3): 848–852. doi:10.1152/japplphysiol.00285.2006. PMID 16741262.
- ↑ Forbes SC, Bell GJ, Turner AJ, Hick PE, Bland RD, Clarke TL, Harden LB (Feb 1976). "Rapid infusion of sodium bicarbonate and albumin into high-risk premature infants soon after birth: a controlled, prospective trial". (review). American Journal of Obstetrics and Gynecology. 124 (3): 405–11. doi:10.1139/h11-035. PMID 21574873.
- ↑ Finsterer J (Nov 2009). "Management of mitochondrial stroke-like-episodes". (review). European Journal of Neurology. 16 (11): 1178–84. doi:10.1111/j.1468-1331.2009.02789.x. PMID 19780807.
- ↑ Nakaki T, Hishikawa K, Suzuki H, Saruta T, Kato R (Sep 1990). "L-arginine-induced hypotension". (primary). Lancet. 336 (8716): 696. doi:10.1016/0140-6736(90)92196-O. PMID 1975886.
- ↑ Gui S, Jia J, Niu X, Bai Y, Zou H, Deng J, Zhou R (Mar 2014). "Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: a systematic review". (review). Journal of the Renin-Angiotensin-Aldosterone System. 15 (1): 88–96. doi:10.1177/1470320313475910. PMID 23435582.
External links
| Wikimedia Commons has media related to arginine. |
- NIST Chemistry Webbook
- Mayo Clinic discussion of Arginine.
- National Institute of Health discussion of Arginine.