Nitroso

Nitroso refers to a functional group in organic chemistry which has the NO group attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds (e.g., nitrosoalkanes; R−N=O), S-nitroso compounds (nitrosothiols; RS−N=O), N-nitroso compounds (e.g., nitrosamines, R1N(−R2)−N=O), and O-nitroso compounds (alkyl nitrites; RO−N=O).
Nitrosyls are non-organic compounds containing the NO group, for example directly bound to the metal via the N atom, giving a metal–NO moiety. Alternatively, a nonmetal example is the common reagent nitrosyl chloride (Cl−N=O).
Nitric oxide is a stable radical, having an unpaired electron.
Reduction of nitric oxide gives the hyponitrite anion, NO−:
- NO + e− → NO−
Oxidation of NO yields the nitrosonium cation, NO+:
- NO → NO+ + e−
Nitrosyl as a ligand

Nitric oxide can serve as a ligand in complexes. The resulting complexes are called metal nitrosyls, and can bond to a metal atom in two extreme modes: as NO+ and as NO−. It is generally assumed that NO+ coordinates linearly, the M−N−O angle being 180°, whereas NO− forms a bent geometry, with an M−N−O angle of approximately 120°. However, the results of many studies have shown that the ionic descriptions of the NO ligand do not correlate with metal–NO geometry. A more realistic description of electron-counting in metal–nitrosyl chemistry is given by the Enemark–Feltham notation.
Organonitroso compounds
Nitroso compounds can be prepared by the reduction of nitro compounds or by the oxidation of hydroxylamines. A good example is (CH3)3CNO, known formally as 2-methyl-2-nitrosopropane, or t-BuNO, which is prepared by the following sequence:[1]
- (CH3)3CNH2 → (CH3)3CNO2
- (CH3)3CNO2 → (CH3)3CNHOH
- (CH3)3CNHOH → (CH3)3CNO
(CH3)3CNO is blue and exists in solution in equilibrium with its dimer, which is colorless, m.p. 80–81 °C.
In the Fischer–Hepp rearrangement aromatic 4-nitrosoanilines are prepared from the corresponding nitrosamines. Another named reaction involving a nitroso compound is the Barton reaction.
Organonitroso compounds serve as a ligands for transition metals.[2]
Nitrosation vs. nitrosylation
Nitrite can enter two kinds of reaction, depending on the physico-chemical environment.
- Nitrosylation is adding a nitrosyl ion NO− to a metal (e.g. iron) or a thiol, leading to nitrosyl iron Fe–NO (e.g., in nitrosylated heme = nitrosylheme) or S-nitrosothiols (RSNOs).
- Nitrosation is adding a nitrosonium ion NO+ to an amine –NH2 leading to a nitrosamine. This conversion occurs at acidic pH, particularly in the stomach, as shown in the equation for the formation of N-phenylnitrosamine:
- NO−
2 + H+ ⇌ HONO - HONO + H+ ⇌ H2O + NO+
- C6H5NH2 + NO+ → C6H5N(H)NO + H+
- NO−
Many primary alkyl N-nitroso compounds, such as CH3N(H)NO, tend to be unstable with respect to hydrolysis to the alcohol. Those derived from secondary amines (e.g., (CH3)2NNO derived from dimethylamine) are more robust. It is these N-nitrosamines that are carcinogens in rodents.
In food
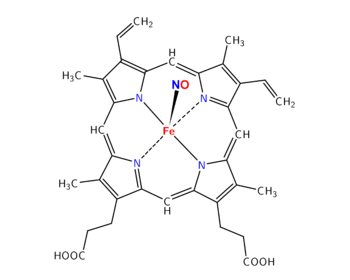
In foodstuffs and in the gastro-intestinal tract, nitrosation and nitrosylation do not have the same consequences on consumer health.
- In cured meat: Meat processed by curing contains nitrite and has a pH of 5 approximately, where almost all nitrite is present as NO−
2 (99%). Cured meat is also added with sodium ascorbate (or erythorbate or vitamin C). As demonstrated by S. Mirvish, ascorbate inhibits nitrosation of amines to nitrosamine, because ascorbate reacts with NO−
2 to form NO.[3][4] Ascorbate and pH 5 thus favor nitrosylation of heme iron, forming nitrosylheme, a red pigment when included inside myoglobin, and a pink pigment when it has been released by cooking. It participates to the "bacon flavor" of cured meat: nitrosylheme is thus considered a benefit for the meat industry and for consumers.[5] - In the stomach: secreted Hydrogen Chloride makes an acidic environment (pH=2) and ingested nitrite (with food or saliva) leads to nitrosation of amines, that yields nitrosamines (potential carcinogens). Nitrosation is low if amine concentration is low (e.g., low-protein diet, no fermented food) or if vitamin C concentration is high (e.g., high fruit diet). Then S-nitrosothiols are formed, that are stable at pH 2.
- In the colon: neutral pH does not favor nitrosation. No nitrosamine is formed in stools, even after addition of a secondary amine or nitrite.[6] Neutral pH favors NO− release from S-nitrosothiols, and nitrosylation of iron. The previously called NOC (N-nitroso compounds) measured by Bingham's team in stools from red meat-fed volunteers[7] were, according to Bingham and Kuhnle, largely non-N-nitroso ATNC (apparent total nitroso compounds), e.g., S-nitrosothiols and nitrosyl iron (as nitrosyl heme).[8]
See also
- Nitrosamine, the functional group with the NO attached to an amine, such as R2N–NO
- Nitrosobenzene
- Nitric oxide
- Nitroxyl
References
- ↑ Calder, A.; Forrester, A. R.; Hepburn, S. P. "2-Methyl-2-nitrosopropane and Its Dimer". Org. Synth. 52: 77.; Coll. Vol., 6,, p. 803
- ↑ Pilato, R. S.; McGettigan, C.; Geoffroy, G. L.; Rheingold, A. L.; Geib, S. J. (1990). "tert-Butylnitroso complexes. Structural characterization of W(CO)5(N(O)Bu-tert) and [CpFe(CO)(PPh3)(N(O)Bu-tert)]+". Organometallics. 9: 312–17. doi:10.1021/om00116a004.
- ↑ "Ascorbate–nitrite reaction: possible means of blocking the formation of carcinogenic N-nitroso compounds". Science. 177 (4043): 65–8. July 1972. Bibcode:1972Sci...177...65M. doi:10.1126/science.177.4043.65. PMID 5041776.
- ↑ "Effects of vitamins C and E on N-nitroso compound formation, carcinogenesis, and cancer". Cancer. 58 (8 Suppl): 1842–50. October 1986. doi:10.1002/1097-0142(19861015)58:8+<1842::aid-cncr2820581410>3.0.co;2-#. PMID 3756808.
- ↑ Honikel, K. O. (2008). "The use an control of nitrate and nitrite for the processing of meat products". Meat Science. 78: 68–76. doi:10.1016/j.meatsci.2007.05.030.
- ↑ "Absence of volatile nitrosamines in human feces". Cancer Res. 41 (10): 3992–4. October 1981. PMID 7285009.
- ↑ "Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer?". Carcinogenesis. 17 (3): 515–23. March 1996. doi:10.1093/carcin/17.3.515. PMID 8631138.
- ↑ "Diet-induced endogenous formation of nitroso compounds in the GI tract". Free Radic. Biol. Med. 43 (7): 1040–7. October 2007. doi:10.1016/j.freeradbiomed.2007.03.011. PMID 17761300.