Vitamin D deficiency
| Vitamin D deficiency | |
|---|---|
|
Calcitriol (1,25-dihydroxycholecalciferol). Active form. Note extra OH groups at upper right and lower right. | |
| Classification and external resources | |
| Specialty | endocrinology |
| ICD-10 | E55 |
| ICD-9-CM | 268 |
| DiseasesDB | 13942 |
| MeSH | D014808 |
Vitamin D deficiency, or Hypovitaminosis D, can result from inadequate nutritional intake of vitamin D and/or inadequate sunlight exposure (in particular sunlight with adequate ultraviolet B rays), disorders limiting vitamin D absorption, and conditions impairing vitamin D conversion into active metabolites—including certain liver, kidney, and hereditary disorders.[1] Deficiency impairs bone mineralization, leading to bone softening diseases as rickets in children and osteomalacia and osteoporosis in adults.[1] Vitamin D deficiency is thought to play a role in the pathogenesis of non-alcoholic fatty liver disease.[2][3]
Classifications
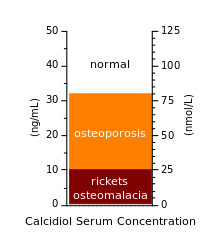
Vitamin D deficiency is typically diagnosed by measuring the concentration of the prehormone calcidiol (25-hydroxyvitamin D) in the blood. which is a precursor to the active form 1,25-dihydroxyvitamin D (calcitriol).[5] One 2008 review has proposed the following four categories for hypovitaminosis D:[6]
- Insufficient 25–74 nmol/L (20–40 ng/mL)
- Normal Range 75–250 nmol/L
These ranges are based on a Canadian range. Season, race and dietary intake affect 25-HydroxyVitamin D levels. Highest levels are found in the summer months and lowest levels during the winter.
Note that 1.0 nmol/L = 0.4 ng/mL for this compound.[7] Other authors have suggested that a 25-hydroxyvitamin D level of 75–80 nmol/L (30–32 ng/mL) may be sufficient[4][5][8] although a majority of healthy young people with comparatively extreme sun exposure did not reach this level in a study done in Hawaii.[9]
Signs and symptoms
Vitamin D deficiency is known to cause several problems,[10] including:
- It has found to be associated with the advancement of cancers, for example; breast, colon, ovarian, and prostate.[11]
- Depression (discussed below)
- Increased risk of fracture- In an elderly population, the incidence of nonvertebral fractures was reduced between 32 and 68% as an effect of vitamin D being supplemented.[11]
- Light-headedness
- Muscle aches and weakness (in particular the limb girdles)[8]
- Muscle twitching (fasciculations) is commonly seen due to reduced ionised calcium, arising from a low vitamin D.[12][13][14]
- Osteomalacia, a bone-thinning disorder that occurs exclusively in adults and is characterized by proximal muscle weakness and bone fragility.
- Osteoporosis, a condition characterized by reduced bone mineral density and increased bone fragility.
- Periodontitis, local inflammatory bone loss that can result in tooth loss[15]
- Rickets, a childhood disease characterized by impeded growth and deformity of the long bones. The earliest sign of subclinical vitamin D deficiency is craniotabes, abnormal softening or thinning of the skull.[16]
The role of diet in the development of rickets was determined by Edward Mellanby between 1918 and 1920.[17] In 1921, Elmer McCollum identified an antirachitic substance found in certain fats that could prevent rickets. Because the newly discovered substance was the fourth vitamin identified, it was called vitamin D.[17] The 1928 Nobel Prize in Chemistry was awarded to Adolf Windaus, who discovered the steroid 7-dehydrocholesterol, the precursor of vitamin D.
Prior to the fortification of milk products with vitamin D, rickets was a major public health problem. In the United States, milk has been fortified with 10 micrograms (400 IU) of vitamin D per quart since the 1930s, leading to a dramatic decline in the number of rickets cases.[18] Vitamin D deficiency can be asymptomatic, but may also affect bone mineralization, resulting in failure to achieve an optimal peak bone mass, which increases fracture risk in both childhood and adult life.[19][20]
Depression
Hypovitaminosis D is a risk factor for depression; some studies have found that low levels of vitamin D are associated with depressed feelings and are found in patients who have been diagnosed with depression.[21] Various studies on trial groups have been conducted to find a correlation between hypovitaminosis D and depression. A study conducted by Lamb et al., (2015) on perinatal depression, examined 126 pregnant women and their levels of vitamin D. In the women with the lower levels of vitamin D, a higher rate of depression was observed.[22] Hypovitaminosis D is also considered a risk factor for the development of depressive symptoms in older persons.[23] One study found low serum vitamin D concentrations in patients with schizophrenia,[24] and the active metabolite of vitamin D3 (calcitriol) acts as a catalyst in glutathione production, and low glutathione levels have been implicated in several mental health disorders. In 2016, a review conducted by Parker et al., looked at articles (most of which were published 2011-2016) that examined the link between vitamin D deficiency and depression. The authors found that "empirical studies appear to provide increasing evidence for an association between vitamin D insufficiency and depression."[25]
Risk factors
Those most likely to be affected by vitamin D deficiency are people with little or no solar exposure. Climate, dress habits, avoiding sun exposure and too much sunscreen protection limit the production of Vitamin D.[26]
Age
The amount of vitamin D recommended for all infants, children, and adolescents has recently increased – from 400 to 600 IU per day. The National Academy of Medicine (NAM) released the Consensus Report on Dietary Reference Intakes for Calcium and Vitamin D on November 30, 2010. The recommendation was for 600 IU of vitamin D a day for those 1-70 and 800 IU for those over 70 years of age.[27] As of October 2008, the American Pediatric Association advises vitamin D supplementation of 400 IU/day (10 μg/d) from birth onwards.[7][28] (1 IU vitamin D is the biological equivalent of 0.025 μg cholecalciferol/ergocalciferol.) The daily dose of 400 IU is required to prevent rickets and possibly also a wide range of chronic nonskeletal diseases.[29] The Canadian Paediatric Society recommends that pregnant or breastfeeding women consider taking 2000 IU/day, that all babies who are exclusively breastfed receive a supplement of 400 IU/day, and that babies living north of 55°N get 800 IU/day from October to April.[30] Health Canada recommends 400IU/day (10 μg/d).[31] Infant formula is generally fortified with vitamin D. Hypovitaminosis D is common in postmenopausal women, regardless of whether they are healthy or have other medical conditions.[32]
Malnutrition
Although rickets and osteomalacia are now rare in Britain, osteomalacia outbreaks in some immigrant communities included women with seemingly adequate daylight outdoor exposure wearing Western clothing.[33] Having darker skin and reduced exposure to sunshine did not produce rickets unless the diet deviated from a Western omnivore pattern characterized by high intakes of meat, fish, and eggs, and low intakes of high-extraction cereals.[34][35][36] The dietary risk factors for rickets include abstaining from animal foods.[37][38] Vitamin D deficiency remains the main cause of rickets among young infants in most countries, because breast milk is low in vitamin D and social customs and climatic conditions can prevent adequate UVB exposure. In sunny countries, such as Nigeria, South Africa, and Bangladesh, where the disease occurs among older toddlers and children, it has been attributed to low dietary calcium intakes, which are characteristic of cereal-based diets with limited access to dairy products.[36] Rickets was formerly a major public health problem among the US population; in Denver, where ultraviolet rays are about 20% stronger than at sea level on the same latitude,[39] almost two-thirds of 500 children had mild rickets in the late 1920s.[40] An increase in the proportion of animal protein[38][41] in the 20th-century American diet coupled with increased consumption of milk[42][43] fortified with relatively small quantities of vitamin D coincided with a dramatic decline in the number of rickets cases.[18]
Obesity
Obese individuals have lower levels of the circulating form of vitamin D, due to the likelihood of decreased bioavailability of vitamin D3 from food and sunlight due to the distribution in adipose tissue.[44] A population-based cohort study in Spain tested 1226 subjects to determine the connection between obesity and hypovitaminosis D; the study reported, "vitamin D deficiency is associated with an increase risk of developing obesity."[45]
Issues regarding treatment
It has been argued that little evidence supports the use of high-dose therapy to attain thresholds for vitamin D deficiency that greatly exceed widely used definitions of vitamin D deficiency (25(OH)D <10 ng/ml or 25 nmol/L), and for vitamin D insufficiency (25(OH)D < 20 ng/ml or 50 nmol/L). Studies are potentially subject to confounding by frailty as people with poorer health are likely to remain indoors, receive less sun exposure, and have low 25(OH)D levels compared to their healthy peers (rather than low vitamin D levels causing ill health). Those leading sedentary lives are at increased risk of obesity, and increased fat mass is inversely associated with 25(OH)D levels.[46][47] This association may confound the reported relationships between low vitamin D status and conditions such as diabetes, ischaemic heart disease, hypertension, and cancer that occur more commonly in obesity.[48] Confounding by health status can be powerful, as evidenced by the disparate results of randomised controlled trials and observational studies of postmenopausal hormone replacement therapy. (see Hormone replacement therapy (menopause)).[49] Obesity remains a likely confounding factor for the associations between low 25(OH)D levels and poor health.[50] Some continue to argue the reverse – that obese and sedentary people are at high risk of many diseases specifically because they have low serum 25(OH)D levels.[51]
Sun exposure
The use of sunscreen with a sun protection factor of 8 can theoretically inhibit more than 95% of vitamin D production in the skin.[18][52] In practice, however, sunscreen is applied so as to have a negligible effect on vitamin D status.[53][54][55] The vitamin D status of those in Australia and New Zealand[56] is unlikely to have been affected by campaigns advocating sunscreen. Instead, wearing clothing is more effective at reducing the amount of skin exposed to UVB and reducing natural vitamin D synthesis. Clothing which covers a large portion of the skin, when worn on a consistent and regular basis, such as the burqa, is correlated with lower vitamin D levels and an increased prevalence of hypovitaminosis D.[57][58]
Regions far from the equator have a high the seasonal variation on the amount and intensity of sunlight. In the UK the prevalence of low vitamin D status in children and adolescents is found to be higher in winter than in summer.[59] Lifestyle factors such as indoor versus outdoor work and time spent in outdoor recreation play an important role.
Habitation and living conditions
Hypovitaminosis D has been associated with urbanisation in terms of both air pollution, which blocks UV light, and an increase in the number of people working indoors. The elderly are generally exposed to less UV light due to hospitalisation, immobility, institutionalisation, and being housebound, leading to decreased levels of vitamin D.[60]
Darker skin color
The reduced pigmentation of light-skinned individuals may result in higher vitamin D levels[4] and that, because melanin acts like a sun-block, dark-skinned individuals, in particular, may require extra vitamin D to avoid deficiency at higher latitudes. Black people are at a higher risk to be vitamin deficient due to their skin color and the melanin levels. The natural selection hypothesis suggests that lighter skin color evolved to optimise vitamin D production in extreme northern and southern latitudes.[61]
Rickets is sometimes due to genetic disorders such as autosomal dominant hypophosphatemic rickets or X-linked hypophosphatemia and associated with consanguineous marriage,[62] and possibly founder effect.[63] In Kashmir, India patients with pseudovitamin D deficiency rickets had grossly raised 25-hydroxyvitamin D concentrations.[64] Skin colour has also been associated with low 25(OH)D, especially in Africans living in countries with a temperate climate. For example, 25-OHD under 10 ng/mL (25 nmol/l) in 44% of asymptomatic East African children living in Melbourne[65][66] However a study of healthy young Ethiopians living in Addis Ababa (10 degrees N) found average 25(OH)D levels of 23.5nmol/L.[67] A review of vitamin D in Africa[68] gives the median levels for equatorial countries: Kenya 65.5 nmol/L and Democratic Republic of the Congo 65nmol/L, concluding that it remains to be established if associations between vitamin D status and health outcomes identified in Western countries can be replicated in African countries.
Vitamin D levels are around 30% higher in northern Europe than in central and southern Europe; higher vitamin D concentrations in northern countries may have a genetic basis.[69][70] In a meta-analysis of cross-sectional studies on serum 25(OH)D concentrations globally, the levels averaged 54 nmol/l and were higher in women than men, and higher in Caucasians than in non-Caucasians. No trend in serum 25(OH)D level was related to latitude.[71] African Americans often have a very low circulating 25(OH)D level. However, those of African descent have higher parathyroid hormone and 1,25-Dihydroxycholecalciferol associated with lower 25-hydroxyvitamin D than other ethnic groups; moreover, they have the greatest bone density[72] and lowest risk of fragility fractures compared to other populations.[73][74][75] Deficiency results in impaired bone mineralization, and leads to bone softening diseases.[76]
Malabsorption
Rates of vitamin D deficiency are higher among people with untreated celiac disease,[77][78] inflammatory bowel disease, exocrine pancreatic insufficiency from cystic fibrosis, and short bowel syndrome,[78]which can all produce poblems of malabsorption.
Cancer
Some evidence suggests hypovitaminosis D may be associated with a worse outcome for some cancers, but evidence is insufficient to recommend that vitamin D be prescribed for people with cancer.[79]
Taking vitamin D supplements has no significant effect on cancer risk.[80] Vitamin D3, however, appears to decrease the risk of death from cancer but concerns with the quality of the data exist.[81]
Pathophysiology
Vitamin D deficiencies are closely related to the development of pre-eclampsia in pregnancy.[82] Vitamin D deficiency leads to impaired intestinal absorption of calcium, which results in decreased levels of serum total and ionized calcium levels. This hypocalcemia gives rise to secondary hyperparathyroidism, which is a homeostatic response aimed at maintaining, initially, serum calcium levels at the expense of the skeleton. Following this parathyroid hormone-induced increase in bone turnover, alkaline phosphatase levels are often increased. PTH not only increases bone resorption, but also leads to decreased urinary calcium excretion while promoting phosphaturia. This results in hypophosphatemia, which exacerbates the mineralization defect in the skeleton.[83] Hypovitaminosis D is linked to the development and severity of depression [84] Maternal vitamin D deficiency may affect the baby, causing overt bone disease from before birth and impairment of bone quality after birth.[20][85]
Diagnosis
The serum concentration of 25(OH)D is typically used to determine vitamin D status. It reflects vitamin D produced in the skin, as well as that acquired from the diet, and has a fairly long circulating half-life of 15 days. It does not, however, reveal the amount of vitamin D stored in other body tissues.
The level of serum 1,25(OH)D is not usually used to determine vitamin D status because it has a short half-life of 15 hours and is tightly regulated by parathyroid hormone, calcium, and phosphate, such that it does not decrease significantly until vitamin D deficiency is already well advanced.
People with a granuloma disease such as sarcoidosis can have a high level of serum 1,25(OH)D, but show a low testing level of serum concentration of 25(OH)D because the granulomas, when active, produce serum 1,25(OH)D. The body is then protecting itself from a calcium dump (high calcium level) by having a low 25(OH)D.[86]
While per mole vitamin D3 is more potent to raise 25(OH)D blood levels than vitamin D2,[87] per IU both D2 and D3 are equal for maintaining 25(OH)D status.[88]
Variability in results of laboratory analyses of the level of 25(OH)D occurs. Falsely low or high values have been obtained depending on the particular test or laboratory used. Beginning in July 2009, a standard reference material became available which should allow laboratories to standardise their procedures.[7]
Some disagreement exists concerning the exact levels of 25(OH)D needed for good health. A level lower than 10 ng/mL (25 nmol/L) is associated with the most severe deficiency diseases: rickets in infants and children, and osteomalacia in adults. A concentration above 15 ng/ml (37.5 nmol/L) is generally considered adequate for those in good health. Levels above 30 ng/ml (75 nmol/L) are proposed by some as desirable for achieving optimum health, but not yet enough evidence exists to support this.[7][89][90]
Levels of 25(OH)D that are consistently above 200 ng/mL (500 nmol/L) are thought to be potentially toxic, although data from humans are sparse. In animal studies, levels up to 400 ng/mL (1,000 nmol/L) were not associated with toxicity.[7] Vitamin D toxicity usually results from taking supplements in excess. Hypercalcemia is typically the cause of symptoms, and levels of 25(OH)D above 150 ng/mL (375 nmol/L) are usually found, although in some cases 25(OH)D levels may appear to be normal. Periodic measurement of serum calcium in individuals receiving large doses of vitamin D is recommended.[1]
In overweight persons, increased fat mass is inversely associated with 25(OH)D levels.[46][47] This association may confound the reported relationships between low vitamin D status and conditions which occur more commonly in obesity[91] as the circulating 25(OH)D underestimates their total body stores.[92] However, as vitamin D is fat-soluble, excess amounts can be stored in fat tissue and used during winter, when sun exposure is limited.[93]
A study of highly sun-exposed (tanned) healthy young skateboarders and surfers in Hawaii found levels below the proposed higher minimum of 30 ng/ml in 51% of the subjects. The highest 25(OH)D concentration was around 60 ng/ml (150nmol/L).[94] A similar <using the same data>study in Hawaii found a range of (11–71 ng/mL) in a population with prolonged extensive skin exposure, while as part of the same study Wisconsin breastfeeding mothers were given supplements. The range of circulating 25(OH)D levels in women in the supplementated group was from 12–77 ng/mL. Levels in the supplemented population in Wisconsin were higher than the sun-exposed group in Hawaii (which again included surfers because it was the same data set).[95]
Another study of African Americans found that blood levels of 25(OH)D decreased linearly with increasing African ancestry, the decrease being 2.5-2.75 nmol/L per 10% increase in African ancestry. Sunlight and diet were 46% less effective in raising these levels among subjects with high African ancestry than among those with low/medium African ancestry.[96] Vitamin-D metabolism possibly differs by ethnicity.[97]
Screening
The usefulness of screening adults without symptoms of vitamin D deficiency is unclear.[98]
Treatment
The replacement of vitamin D needs for treating Vitamin D deficiency depends on the severity of the deficiency. Treatment involves an initial high-dosage treatment phase until the required serum levels are reached, followed by the maintenance of the acquired levels. The lower the 25(OH)D serum concentration is before treatment, the higher is the dosage that is needed in order to quickly reach an acceptable serum level.
The initial high-dosage treatment can be given on a daily or weekly basis or can be given in form of one or several single doses (also known as stoss therapy, from the German word "Stoß" meaning push).[99]
Therapy prescriptions vary, and there is no consensus yet on how best to arrive at an optimum serum level.
Initial phase
Daily or weekly dose
For treating rickets, the American Academy of Pediatrics (AAP) has recommended that pediatric patients receive an initial two- to three-month treatment of “high-dose” vitamin D therapy. In this regime, the daily dosis of cholecalciferol is 1,000 IU for newborns, 1,000 to 5,000 IU for 1- to 12-months old infants, and 5,000 IU for patients over 1 year of age.[99]
For adults, other dosages have been called for. A review of 2008/2009 recommended dosages of 1,000 IU cholecalciferol per 10 nmol/l required serum increase, to be given daily over two to three months.[100] In another proposed cholecalciferol loading dose guideline for vitamin D-deficient adults, a weekly dosage is given, up to a total amount that is proportional to the required serum increase (up to the level of 75 nml/l) and, within certain body weight limits, to body weight.[101]
Single-dose therapy
Alternatively, a single-dose therapy is used for instance if there are concerns regarding the patient's compliance. The single-dose therapy can be given as an injection, but is normally given in form of an oral medication.[99]
Maintenance phase
Once the desired serum levels has been achieved, be it by a high daily or weekly dose or by a single-dose therapy, the AAP recommendation calls for a maintenance supplementation of 400 IU for all age groups, with this dosage being doubled for premature infants, dark-skinned infants and children, children who reside in areas of limited sun exposure (>37.5° latitude), obese patients, and those on certain medications.
Special cases
To maintain blood levels of calcium, therapeutic vitamin D doses are sometimes administered (up to 100,000 IU or 2.5 mg daily) to patients who have had their parathyroid glands removed (most commonly kidney dialysis patients who have had tertiary hyperparathyroidism, but also to patients with primary hyperparathyroidism) or with hypoparathyroidism.[102] Patients with chronic liver disease or intestinal malabsorption disorders may also require larger doses of vitamin D (up to 40,000 IU or 1 mg (1000 micrograms) daily).
See also
References
- 1 2 3 Vitamin D at Merck Manual of Diagnosis and Therapy Professional Edition
- ↑ Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, Koteish AA, Clark JM, Guallar E, Hernaez R (2013). "Meta-analysis: Vitamin D and non-alcoholic fatty liver disease". Alimentary Pharmacology & Therapeutics. 38 (3): 246–54. doi:10.1111/apt.12377. PMID 23786213.
- ↑ Wang, X; Li, W; Zhang, Y; Yang, Y; Qin, G (2015). "Association between vitamin D and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: results from a meta-analysis.". International journal of clinical and experimental medicine. 8 (10): 17221–34. PMID 26770315.
- 1 2 3 Heaney RP (December 2004). "Functional indices of vitamin D status and ramifications of vitamin D deficiency". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1706S–9S. PMID 15585791.
- 1 2 Holick MF (2007). "Vitamin D Deficiency". New England Journal of Medicine. 357 (3): 266–81. doi:10.1056/NEJMra070553. PMID 17634462.
- ↑ Stroud ML, Stilgoe S, Stott VE, Alhabian O, Salman K (December 2008). "Vitamin D – a review". Australian Family Physician. 37 (12): 1002–5. PMID 19142273.
- 1 2 3 4 5 "Dietary Supplement Fact Sheet: Vitamin D". National Institutes of Health. Archived from the original on 2007-09-10. Retrieved 2007-09-10.
- 1 2 "Vitamin D deficiency in adults". Australian Prescriber (33): 103–6. 2010.
- ↑ Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK (2007). "Low Vitamin D Status despite Abundant Sun Exposure". The Journal of Clinical Endocrinology & Metabolism. 92 (6): 2130–5. doi:10.1210/jc.2006-2250. PMID 17426097.
- ↑ Grant WB, Holick MF (June 2005). "Benefits and requirements of vitamin D for optimal health: a review" (PDF). Alternative Medicine Review. 10 (2): 94–111. PMID 15989379.
- 1 2 Cherniack; Levis; Troen (2008). "Hypovitaminosis D: a widespread epidemic". Geriatrics. Retrieved 14 April 2015.
- ↑ Reid, P.G. (2012). "Acute Management of Calcium Disorders". Topics in Companion Animal Medicine. 27: 167–171.
- ↑ Madson, D.M., (2012). "Rickets case series and diagnostic review of hypovitaminosis D in swine". Journal of Veterinary Diagnostic Investigation. 24 (6): 1137–1144. doi:10.1177/1040638712461487.
- ↑ Holick, M.F. (2008). "Rickets case series and diagnostic review of hypovitaminosis D in swine". Nutrition Reviews. 66 (2): S182–S194. doi:10.1111/j.1753-4887.2008.00104.x.
- ↑ Wang, Chin-Wei; McCauley, Laurie K. (30 September 2016). "Osteoporosis and Periodontitis". Current Osteoporosis Reports. doi:10.1007/s11914-016-0330-3. PMID 27696284.
- ↑ Yorifuji J, Yorifuji T, Tachibana K, Nagai S, Kawai M, Momoi T, Nagasaka H, Hatayama H, Nakahata T (2008). "Craniotabes in Normal Newborns: The Earliest Sign of Subclinical Vitamin D Deficiency". The Journal of Clinical Endocrinology & Metabolism. 93 (5): 1784–8. doi:10.1210/jc.2007-2254. PMID 18270256.
- 1 2 Rajakumar K (August 2003). "Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective". Pediatrics. 112 (2): e132–5. doi:10.1542/peds.112.2.e132. PMID 12897318.
- 1 2 3 Holick MF (December 2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1678S–88S. PMID 15585788.
- ↑ Winzenberg T, Jones G (2013). "Vitamin D and bone health in childhood and adolescence". Calcif Tissue Int (Review). 92 (2): 140–50. doi:10.1007/s00223-012-9615-4. PMID 22710658.
- 1 2 Elidrissy AT (2016). "The Return of Congenital Rickets, Are We Missing Occult Cases?". Calcif Tissue Int (Review). 99 (3): 227–36. doi:10.1007/s00223-016-0146-2. PMID 27245342.
- ↑ Robert & Howland, H & MD (2011). "Vitamin D and Depression". Journal of Psychosocial Nursing and Mental Health Services. 49: 15–18. doi:10.3928/02793695-20110111-02. Retrieved 15 April 2015.
- ↑ Lamb, Hobel, Pepkowitz, Holmquist, Young, Wallston & Lutenbacher., Amy, Calvin, Sam, Brett, Donnabeth, Ken & Melanie (January 2015). "Vitamin D deficiency and depressive symptoms in the perinatal period: a prospective study". American Journal of Obstetrics & Gynecology. 212: S371. doi:10.1016/j.ajog.2014.10.969. Retrieved 15 April 2015.
- ↑ Milaneschi Y, Shardell M, Corsi AM, Vazzana R, Bandinelli S, Guralnik JM, Ferrucci L (2010). "Serum 25-Hydroxyvitamin D and Depressive Symptoms in Older Women and Men". The Journal of Clinical Endocrinology & Metabolism. 95 (7): 3225–33. doi:10.1210/jc.2010-0347. PMID 20444911.
- ↑ Itzhaky D, Amital D, Gorden K, Bogomolni A, Arnson Y, Amital H (2012). "Low serum vitamin D concentrations in patients with schizophrenia". The Israel Medical Association journal : IMAJ. 14 (2): 88–92. PMID 22693787.
- ↑ Parker, GB; Brotchie, H; Graham, RK (11 October 2016). "Vitamin D and depression". Journal of affective disorders. 208: 56–61. doi:10.1016/j.jad.2016.08.082. PMID 27750060.
- ↑ Kennel, Kurt, MD et al., Vitamin D Deficiency in Adults: When to Test and How to Treat, Mayo Clinic Proceedings, August 2010, pp752–758
- ↑ http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx
- ↑ Kingsbury, Kathleen (2008-10-13). "Kids Aren't Getting Enough Vitamin D". Time Health & Science. Time Inc. Retrieved 15 November 2008.
- ↑ Greer F (2009). "Recommended vitamin D intake in children: reasons for the recent increase". Consultant for Pediatricians. 8 (9): 323–329.
- ↑ Canadian mothers and babies don't get enough vitamin D 2007 Canadian Paediatric Society Recommendation
- ↑ Vitamin D Supplementation for Breastfed Infants – 2004 Health Canada Recommendation
- ↑ Mosoni A.M.; Menoyo I.; Bocanera R.; Pezzotto S.M.; Morosano M.E. (2014). "Hypovitaminosis D and associated risk factors in postmenopausal women". Health. 6 (11): 1180–1190.
- ↑ Dunnigan MG, Henderson JB (2007). "An epidemiological model of privational rickets and osteomalacia". Proceedings of the Nutrition Society. 56 (3): 939–56. doi:10.1079/PNS19970100. PMID 9483661.
- ↑ Robertson I, Ford JA, McIntosh WB, Dunnigan MG (2007). "The role of cereals in the aetiology of nutritional rickets: The lesson of the Irish National Nutrition Survey 1943–8". British Journal of Nutrition. 45 (1): 17–22. doi:10.1079/BJN19810073. PMID 6970590.
- ↑ Clements, M. R. (1989). "The problem of rickets in UK Asians". Journal of Human Nutrition and Dietetics. 2 (2): 105–116. doi:10.1111/j.1365-277X.1989.tb00015.x.
- 1 2 Pettifor JM (2004). "Nutritional rickets: Deficiency of vitamin D, calcium, or both?". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1725S–9S. PMID 15585795.
- ↑ Dunnigan MG, Henderson JB (1997). "An epidemiological model of privational rickets and osteomalacia". The Proceedings of the Nutrition Society. 56 (3): 939–56. doi:10.1079/PNS19970100. PMID 9483661.
- 1 2 Dunnigan MG, Henderson JB, Hole DJ, Barbara Mawer E, Berry JL (2007). "Meat consumption reduces the risk of nutritional rickets and osteomalacia". British Journal of Nutrition. 94 (6): 983–91. doi:10.1079/BJN20051558. PMID 16351777.
- ↑ US National Institutes Of Health, National cancer Institute
- ↑ Weick MT (1967). "A history of rickets in the United States". The American Journal of Clinical Nutrition. 20 (11): 1234–41. PMID 4862158.
- ↑ Garrison, R., Jr., Somer, E., The nutrition desk reference(1997)
- ↑ DuPuis, E. Melanie (2002). Nature's Perfect Food: How Milk Became America's Drink. ISBN 978-0-8147-1938-1.
- ↑ Teegarden D, Lyle RM, Proulx WR, Johnston CC, Weaver CM (1999). "Previous milk consumption is associated with greater bone density in young women". The American Journal of Clinical Nutrition. 69 (5): 1014–7. PMID 10232644.
- ↑ Wortsman, J., Matsuoka, L. Y., Chen, T. C., Lu, Z., & Holick, M. F, J, Y, C, Z & F (2000). "Decreased bioavailability of vitamin D in obesity". The American Journal of Clinical Nutrition. Retrieved 15 April 2015.
- ↑ Gonzalez, Rojo, Morcillo, Gutierrez-Repiso,Rubio, E & Soriguer. (2 August 2014). "Vitamin D deficiency and obesity" (PDF). Atherosclerosis. 235: e212. doi:10.1016/j.atherosclerosis.2014.05.627. Retrieved 15 April 2015.
- 1 2 Lucas JA, Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR (2005). "Determinants of vitamin D status in older women living in a subtropical climate". Osteoporosis International. 16 (12): 1641–8. doi:10.1007/s00198-005-1888-2. PMID 16027959.
- 1 2 Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR (2006). "Determinants of vitamin D status in older men living in a subtropical climate". Osteoporosis International. 17 (12): 1742–8. doi:10.1007/s00198-006-0190-2. PMID 16932872.
- ↑ Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA (2001). "Impact of Overweight on the Risk of Developing Common Chronic Diseases During a 10-Year Period". Archives of Internal Medicine. 161 (13): 1581–6. doi:10.1001/archinte.161.13.1581. PMID 11434789.
- ↑ Bolland MJ, Grey A, Cundy T, Reid IR (2007). "Defining vitamin D deficiency". The New Zealand medical journal. 120 (1263): U2760. PMID 17972977.
- ↑ Reddy KK, Gilchrest BA (2010). "What is All This Commotion about Vitamin D?". Journal of Investigative Dermatology. 130 (2): 321–6. doi:10.1038/jid.2009.353. PMID 20081879.
- ↑ Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC (2007). "Vitamin D and prevention of breast cancer: Pooled analysis". The Journal of Steroid Biochemistry and Molecular Biology. 103 (3–5): 708–11. doi:10.1016/j.jsbmb.2006.12.007. PMID 17368188.
- ↑ Sayre RM, Dowdy JC (2007). "Darkness at Noon: Sunscreens and Vitamin D3". Photochemistry and Photobiology. 83 (2): 459–63. doi:10.1562/2006-06-29-RC-956. PMID 17115796.
- ↑ Marks R, Foley PA, Jolley D, Knight KR, Harrison J, Thompson SC (1995). "The Effect of Regular Sunscreen Use on Vitamin D Levels in an Australian Population". Archives of Dermatology. 131 (4): 415–21. doi:10.1001/archderm.1995.01690160043006. PMID 7726582.
- ↑ Farrerons J, Barnadas M, Rodríguez J, Renau A, Yoldi B, López-Navidad A, Moragas J (1998). "Clinically prescribed sunscreen (sun protection factor 15) does not decrease serum vitamin D concentration sufficiently either to induce changes in parathyroid function or in metabolic markers". British Journal of Dermatology. 139 (3): 422–7. doi:10.1046/j.1365-2133.1998.02405.x. PMID 9767286.
- ↑ Norval M, Wulf HC (2009). "Does chronic sunscreen use reduce vitamin D production to insufficient levels?". British Journal of Dermatology. 161 (4): 732–6. doi:10.1111/j.1365-2133.2009.09332.x. PMID 19663879.
- ↑ Nowson CA, Margerison C (August 2002). "Vitamin D intake and vitamin D status of Australians". The Medical Journal of Australia. 177 (3): 149–52. PMID 12149085.
- ↑ Mishal AA (2001). "Effects of Different Dress Styles on Vitamin D Levels in Healthy Young Jordanian Women". Osteoporosis International. Springer London. 12 (11): 931–935. doi:10.1007/s001980170021. PMID 11804019.
The prevalence of hypovitaminosis D was 62.3% in the study groups as a whole. Dress styles covering the whole body, totally or nearly totally, have adverse effects on 25(OH)D levels and may produce a state of secondary hyperparathyroidism on the long run. Although Jordan enjoys plenty of sunshine, these data are suggestive of widespread hypovitaminosis D in Jordan.
- ↑ Bandgar, TR; NS Shah. "Vitamin D and Hip Fractures: Indian Scenario". Journal of the Association of Physicians of India. 58 (September 2010). Retrieved 2010-09-15.
Social and religious customs that require people to wear concealing clothing, veiling and traditional attire, such as the burqa, salvar kameez, and sari significantly prevents sun exposure.
- ↑ Cashman KD (2007). "Vitamin D in childhood and adolescence". Postgraduate Medical Journal (Review). 83 (978): 230–5. doi:10.1136/pgmj.2006.052787. PMC 2600028
 . PMID 17403948.
. PMID 17403948. - ↑ Mithal; Wahl; Bonjour; Burckhardt; Dawson-Hughes; El-Hajj Fuleihan (2009). "Global vitamin D status and determinants of hypovitaminosis D". Osteoporosis International. 20: 1807–1820. doi:10.1007/s00198-009-0954-6. Retrieved 2015-04-12. Missing
|last6=in Authors list (help) - ↑ Yuen AW, Jablonski NG (January 2010). "Vitamin D: in the evolution of human skin colour". Medical Hypotheses. 74 (1): 39–44. doi:10.1016/j.mehy.2009.08.007. PMID 19717244.
- ↑ Sibert JR, Moffat WM (1973). "Hereditary pseudo vitamin D deficiency rickets in a Pakistani infant". Archives of Disease in Childhood. 48 (10): 814–6. doi:10.1136/adc.48.10.814. PMC 1648552
 . PMID 4542997.
. PMID 4542997. - ↑ Labuda M, Labuda D, Korab-Laskowska M, Cole DE, Zietkiewicz E, Weissenbach J, Popowska E, Pronicka E, Root AW, Glorieux FH (1996). "Linkage disequilibrium analysis in young populations: pseudo-vitamin D-deficiency rickets and the founder effect in French Canadians". American Journal of Human Genetics. 59 (3): 633–43. PMC 1914903
 . PMID 8751865.
. PMID 8751865. - ↑ Zargar AH, Mithal A, Wani AI, Laway BA, Masoodi SR, Bashir MI, Ganie MA (2000). "Pseudovitamin D deficiency rickets – a report from the Indian subcontinent". Postgraduate Medical Journal. 76 (896): 369–72. doi:10.1136/pmj.76.896.369. PMC 1741602
 . PMID 10824056.
. PMID 10824056. - ↑ Benson J, Skull S (2007). "Hiding from the sun – vitamin D deficiency in refugees". Australian family physician. 36 (5): 355–7. PMID 17492073.
- ↑ McGillivray G, Skull SA, Davie G, Kofoed SE, Frydenberg A, Rice J, Cooke R, Carapetis JR (2007). "High prevalence of asymptomatic vitamin D and iron deficiency in East African immigrant children and adolescents living in a temperate climate". Archives of Disease in Childhood. 92 (12): 1088–93. doi:10.1136/adc.2006.112813. PMC 2066069
 . PMID 17768148.
. PMID 17768148. - ↑ Feleke Y, Abdulkadir J, Mshana R, Mekbib TA, Brunvand L, Berg JP, Falch JA (1999). "Low levels of serum calcidiol in an African population compared to a North European population". European Journal of Endocrinology. 141 (4): 358–60. doi:10.1530/eje.0.1410358. PMID 10526248.
- ↑ Prentice A, Schoenmakers I, Jones KS, Jarjou LM, Goldberg GR (2009). "Vitamin D Deficiency and Its Health Consequences in Africa". Clinical Reviews in Bone and Mineral Metabolism. 7: 94–106. doi:10.1007/s12018-009-9038-6. PMID 25110467.
- ↑ Snellman G, Melhus H, Gedeborg R, Olofsson S, Wolk A, Pedersen NL, Michaëlsson K (2009). Bochdanovits Z, ed. "Seasonal Genetic Influence on Serum 25-Hydroxyvitamin D Levels: A Twin Study". PLoS ONE. 4 (11): e7747. Bibcode:2009PLoSO...4.7747S. doi:10.1371/journal.pone.0007747. PMC 2774516
 . PMID 19915719.
. PMID 19915719. - ↑ Lips P (2007). "Vitamin D status and nutrition in Europe and Asia". The Journal of Steroid Biochemistry and Molecular Biology. 103 (3–5): 620–5. doi:10.1016/j.jsbmb.2006.12.076. PMID 17287117.
- ↑ Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, Vestergaard P (2008). "Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis". Osteoporosis International. 20 (1): 133–40. doi:10.1007/s00198-008-0626-y. PMID 18458986.
- ↑ Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA (2002). "Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors". The Journal of Clinical Endocrinology and Metabolism. 87 (7): 3057–67. doi:10.1210/jc.87.7.3057. PMID 12107201.
- ↑ Harris SS (2006). "Vitamin D and African Americans". The Journal of Nutrition. 136 (4): 1126–9. PMID 16549493.
- ↑ Gadegbeku CA, Chertow GM (2009). "Cum Hoc, Ergo Propter Hoc: Health Disparities Real and Imagined". Clinical Journal of the American Society of Nephrology. 4 (2): 251–3. doi:10.2215/CJN.06361208. PMID 19201919.
- ↑ Aloia JF (2008). "African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox". The American Journal of Clinical Nutrition. 88 (2): 545S–550S. PMC 2777641
 . PMID 18689399.
. PMID 18689399. - ↑ Grant WB, Holick MF (2005). "Benefits and requirements of vitamin D for optimal health: a review". Alternative medicine review. 10 (2): 94–111. PMID 15989379.
- ↑ Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G (2013). "Appropriate nutrient supplementation in celiac disease". Ann Med (Review). 45 (8): 522–31. doi:10.3109/07853890.2013.849383. PMID 24195595.
- 1 2 Margulies SL, Kurian D, Elliott MS, Han Z (2015). "Vitamin D deficiency in patients with intestinal malabsorption syndromes--think in and outside the gut.". J Dig Dis (Review). 16 (11): 617–33. doi:10.1111/1751-2980.12283. PMID 26316334.
- ↑ Buttigliero C, Monagheddu C, Petroni P, Saini A, Dogliotti L, Ciccone G, Berruti A (2011). "Prognostic Role of Vitamin D Status and Efficacy of Vitamin D Supplementation in Cancer Patients: A Systematic Review". The Oncologist. 16 (9): 1215–27. doi:10.1634/theoncologist.2011-0098. PMC 3228169
 . PMID 21835895.
. PMID 21835895. - ↑ Bolland, Mark J; Grey, Andrew; Gamble, Greg D; Reid, Ian R (2014). "The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: A trial sequential meta-analysis". The Lancet Diabetes & Endocrinology. 2 (4): 307–320. doi:10.1016/S2213-8587(13)70212-2. PMID 24703049.
- ↑ Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C (2014). "Vitamin D supplementation for prevention of mortality in adults". Cochrane Database of Systematic Reviews. 1: CD007470. doi:10.1002/14651858.cd007470.pub3. PMID 24414552.
- ↑ Xu L.; Lee M.; Jeyabalan A.; Roberts J.M. (2014). "The relationship of hypovitaminosis D and IL-6 in preeclampsia". American Journal of Obstetrics and Gynaecology. 210 (2): 149–149.
- ↑ Longo, Dan L.; Fauci, Anthony; Kasper, Dennis; Hauser, Stephen; Jameson, J. Jerry; Loscalzo, Joseph. Harrison's Principles of Internal Medicine (18th ed.). p. 3094.
- ↑ Milaneschi Y.; Hoogendijk W.; Lips P.; Heijboer A.C.; Schoevers R.; Van Hemert A.M.; Penninx B.W.J.H. (2012). "The association between low vitamin D and depressive disorders". Molecular Psychiatry. 19 (4): 444–444. doi:10.1038/mp.2013.36.
- ↑ Paterson CR, Ayoub D (2015). "Congenital rickets due to vitamin D deficiency in the mothers.". Clin Nutr (Review). 34 (5): 793–8. doi:10.1016/j.clnu.2014.12.006. PMID 25552383.
- ↑ http://www.vitamindwiki.com/Sarcoidosis+%28rare%29+problems+when+taking+vitamin+D%20[]
- ↑ Heaney RP, Recker RR, Grote J, Horst RL, Armas LA (2011). "Vitamin D3Is More Potent Than Vitamin D2in Humans". The Journal of Clinical Endocrinology & Metabolism. 96 (3): E447. doi:10.1210/jc.2010-2230. PMID 21177785.
- ↑ Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD (2008). "Vitamin D2 is as Effective as Vitamin D3 in Maintaining Circulating Concentrations of 25-Hydroxyvitamin D". The Journal of Clinical Endocrinology and Metabolism. 93 (3): 677–681. doi:10.1210/jc.2007-2308. PMC 2266966
 . PMID 18089691.
. PMID 18089691. - ↑ Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM (2010). "Vitamin D and Cardiometabolic Outcomes: A Systematic Review". Annals of Internal Medicine. 152 (5): 307–314. doi:10.7326/0003-4819-152-5-201003020-00009. PMC 3211092
 . PMID 20194237.
. PMID 20194237. - ↑ Wang L, Manson JE, Song Y, Sesso HD (2010). "Systematic Review: Vitamin D and Calcium Supplementation in Prevention of Cardiovascular Events". Annals of Internal Medicine. 152 (5): 315–23. doi:10.7326/0003-4819-152-5-201003020-00010. PMID 20194238.
- ↑ Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA (2001). "Impact of Overweight on the Risk of Developing Common Chronic Diseases During a 10-Year Period". Archives of Internal Medicine. 161 (13): 1581–6. doi:10.1001/archinte.161.13.1581. PMID 11434789.
- ↑ Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000). "Decreased bioavailability of vitamin D in obesity". The American Journal of Clinical Nutrition. 72 (3): 690–3. PMID 10966885.
- ↑ Alpert PT, Shaikh U (2007). "The effects of Vitamin D Deficiency and Insufficiency on the Endocrine and Paracrine Systems". Biological Research for Nursing. 9 (2): 11–129. doi:10.1177/1099800407308057. PMID 17909164.
- ↑ Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK (2007). "Low vitamin D status despite abundant sun exposure". The Journal of Clinical Endocrinology and Metabolism. 92 (6): 2130–5. doi:10.1210/jc.2006-2250. PMID 17426097.
- ↑ Hollis BW, Wagner CL, Drezner MK, Binkley NC (2007). "Circulating Vitamin D3 and 25-hydroxyvitamin D in Humans: An Important Tool to Define Adequate Nutritional Vitamin D Status". The Journal of Steroid Biochemistry and Molecular Biology. 103 (3–5): 631–4. doi:10.1016/j.jsbmb.2006.12.066. PMC 1868557
 . PMID 17218096.
. PMID 17218096. - ↑ Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, Hargreaves MK, Hollis BW, Blot WJ (2010). "Blood vitamin D levels in relation to genetic estimation of African ancestry". Cancer Epidemiology, Biomarkers & Prevention. 19 (9): 2325–31. doi:10.1158/1055-9965.EPI-10-0482. PMC 2938736
 . PMID 20647395.
. PMID 20647395. - ↑ Freedman DM, Looker AC, Chang SC, Graubard BI (2007). "Prospective study of serum vitamin D and cancer mortality in the United States". Journal of the National Cancer Institute. 99 (21): 1594–602. doi:10.1093/jnci/djm204. PMID 17971526.
- ↑ LeFevre ML (25 November 2014). "Screening for Vitamin D Deficiency in Adults: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine. 162: 133–40. doi:10.7326/M14-2450. PMID 25419853.
- 1 2 3 Lee JY, So TY, Thackray J (2013). "A review on vitamin d deficiency treatment in pediatric patients". The Journal of Pediatric Pharmacology and Therapeutics : JPPT : the Official Journal of PPAG (Review). 18 (4): 277–91. doi:10.5863/1551-6776-18.4.277. PMC 3979050
 . PMID 24719588.
. PMID 24719588. - ↑ Moyad MA (2009). "Vitamin D: a rapid review". Dermatology Nursing / Dermatology Nurses' Association. 21 (1): 25–30, 55. PMID 19283958. Section Dosage of Vitamin D Needed To Achieve 35 to 40 ng/ml (90–100 nmol/L). Re-published from Moyad MA (2008). "Vitamin D: a rapid review". Urologic Nursing (Review). 28 (5): 343–9, 384; quiz 350. PMID 18980100.
- ↑ van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H (April 2010). "Cholecalciferol loading dose guideline for vitamin D-deficient adults". Eur. J. Endocrinol. 162 (4): 805–11. doi:10.1530/EJE-09-0932. PMID 20139241.
- ↑ Holick MF (November 2005). "The vitamin D epidemic and its health consequences". The Journal of Nutrition. 135 (11): 2739S–48S. PMID 16251641.
External links
- VITAMIN D DEFICIENCY – Treatment and diagnosis from UCTV (University of California) (videos)
- Vitamin D Council
- "The Power of D", Nathan Seppa, Science News, July 16, 2011, pages 22–26, a review article.

