Pellagra
| Pellagra | |
|---|---|
 | |
| Person with pellagra with typical skin lesions | |
| Classification and external resources | |
| Specialty | Dermatology |
| ICD-10 | E52 |
| ICD-9-CM | 265.2 |
| DiseasesDB | 9730 |
| MedlinePlus | 000342 |
| eMedicine | ped/1755 |
| Patient UK | Pellagra |
| MeSH | C18.654.521.500.133.699.529 |
Pellagra is a vitamin deficiency disease most frequently caused by a chronic lack of niacin (vitamin B3 or vitamin PP, from pellagra-preventing factor) in the diet. It can be caused by decreased intake of niacin or tryptophan,[1] and possibly by excessive intake of leucine.[2] It may also result from alterations in protein metabolism in disorders such as carcinoid syndrome or Hartnup disease. A deficiency of the amino acid lysine can lead to a deficiency of niacin, as well.[3]
Signs and symptoms
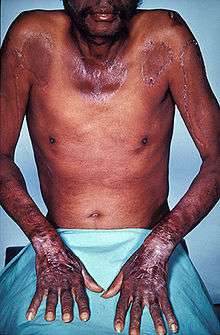
Pellagra is classically described by "the three Ds": diarrhea, dermatitis, dementia.[4] A more comprehensive list of symptoms includes:
- High sensitivity to sunlight
- Aggression
- Dermatitis, alopecia (hair loss), edema (swelling)
- Smooth, beefy red glossitis (tongue inflammation)
- Red skin lesions
- Insomnia
- Weakness
- Mental confusion
- Ataxia (lack of coordination), paralysis of extremities, peripheral neuritis (nerve damage)
- Diarrhea
- Dilated cardiomyopathy (enlarged, weakened heart)
- Eventually dementia
J. Frostig and Tom Spies (acc. to Cleary and Cleary) described more specific psychological symptoms of pellagra as:[5][6]
- Psychosensory disturbances (impressions as being painful, annoying bright lights, odors intolerance causing nausea and vomiting, dizziness after sudden movements)
- Psychomotor disturbances (restlessness, tense and a desire to quarrel, increased preparedness for motor action)
- Emotional disturbances
Despite clinical symptoms, blood level of tryptophan or urinary metabolites such as 2-pyridone/N-methylniacinamide ratio <2 or NAD/NADP ratio in red blood cells could be used to diagnose pellagra. Diagnosis could be confirmed after rapid improvements in the symptoms in patients using high doses of niacin (50–500 mg/day) or niacin enriched food.[7]
Pathophysiology
Pellagra can develop according to several mechanisms, classically as a result of niacin (vitamin B3) deficiency, which results in decreased NAD production leading to most of the pathology (since NAD and its phosphorylated NADP form are cofactors required in many body processes, the pathological impact of pellagra is broad and results in death if not treated).
The first mechanism is simple dietary lack of niacin. Second, it may result from deficiency of tryptophan,[1] an essential amino acid found in meat, poultry, fish, eggs, and peanuts[8] that the body converts into niacin. Third, it may be caused by excess leucine, as it inhibits quinolinate phosphoribosyl transferase (QPRT) and inhibits the formation of Niacin or Nicotinic acid to Nicotinamide mononucleotide (NMN) causing pellegra like symptoms to occur.[2]
Some conditions can prevent the absorption of dietary niacin or tryptophan and lead to pellagra. Inflammation of the jejunum or ileum can prevent nutrient absorption, leading to pellagra, and this can in turn be caused by Crohn's disease.[9] Gastroenterostomy can also cause pellagra.[9] Chronic alcoholism can also cause poor absorption which combines with a diet already low in niacin and tryptophan to produce pellagra.[9] Hartnup disease is a genetic disorder that reduces tryptophan absorption, leading to pellagra.
Alterations in protein metabolism may also produce pellagra-like symptoms. An example is carcinoid syndrome, a disease in which neuroendocrine tumors along the GI tract use tryptophan as the source for serotonin production, which limits the available tryptophan for niacin synthesis. In normal patients, only one percent of dietary tryptophan is converted to serotonin; however, in patients with carcinoid syndrome, this value may increase to 70%. Carcinoid syndrome thus may produce niacin deficiency and clinical manifestations of pellagra. Anti-tuberculosis medication tends to bind to vitamin B6 and reduce niacin synthesis, since B6 (aka pyridoxine) is a required cofactor in the tryptophan-to-niacin reaction.
Several therapeutic drugs can provoke pellagra. These include the antibiotics isoniazid, which decreases available B6 by binding to it and making it inactive, so it cannot be used in niacin synthesis,[10] and chloramphenicol; the anti-cancer agent fluorouracil; and the immunosuppressant mercaptopurine.[9]
Treatment
If untreated, pellagra can kill within four or five years. Treatment is with nicotinamide, which has the same vitamin function as niacin and a similar chemical structure, but has lower toxicity. The frequency and amount of nicotinamide administered depends on the degree to which the condition has progressed.
Epidemiology
Pellagra can be common in people who obtain most of their food energy from maize, notably rural South America, where maize is a staple food. If maize is not nixtamalized, it is a poor source of tryptophan, as well as niacin. Nixtamalization corrects the niacin deficiency, and is a common practice in Native American cultures that grow corn. Following the corn cycle, the symptoms usually appear during spring, increase in the summer due to greater sun exposure, and return the following spring. Indeed, pellagra was once endemic in the poorer states of the U.S. South, such as Mississippi and Alabama, where its cyclical appearance in the spring after meat-heavy winter diets led to it being known as "spring sickness" (particularly when it appeared among more vulnerable children), as well as among the residents of jails and orphanages as studied by Dr. Joseph Goldberger.[11]
Pellagra is common in Africa, Indonesia, and China. In affluent societies, a majority of patients with clinical pellagra are poor, homeless, alcohol-dependent, or psychiatric patients who refuse food.[12] Pellagra was common among prisoners of Soviet labor camps (the Gulag). In addition, pellagra, as a micronutrient deficiency disease, frequently affects populations of refugees and other displaced people due to their unique, long-term residential circumstances and dependence on food aid. Refugees typically rely on limited sources of niacin provided to them, such as groundnuts; the instability in the nutritional content and distribution of food aid can be the cause of pellagra in displaced populations. In the 2000s, there were outbreaks in countries such as Angola, Zimbabwe and Nepal.[13][14][15] In Angola specifically, recent reports show a similar incidence of pellagra since 2002 with clinical pellagra in 0.3% of women and 0.2% of children and niacin deficiency in 29.4% of women and 6% of children related to high untreated corn consumption.[15]
In other countries such as the Netherlands and Denmark, even with sufficient intake of niacin, cases have been reported. In this case deficiency might happen not just because of poverty or malnutrition but secondary to alcoholism, drug interaction (psychotropic, cytostatic, tuberclostatic or analgesics), HIV, vitamin B2 and B6 deficiency, or malabsorption syndromes such as Hartnup and carcinoid.[15][16][17][18][19][20][21][22]
History
The traditional food preparation method of maize ("corn"), nixtamalization, by native New World cultivators who had domesticated corn, required treatment of the grain with lime, an alkali. The lime treatment has been shown to make niacin nutritionally available and reduce the chance of developing pellagra.[23] When maize cultivation was adopted worldwide, this preparation method was not accepted because the benefit was not understood. The original cultivators, often heavily dependent on maize, did not suffer from pellagra; it became common only when maize became a staple that was eaten without the traditional treatment.
Pellagra was first described for its dermatological effect in Spain in 1735 by Gaspar Casal. He explained that the disease causes dermatitis in exposed skin areas such as hands, feet and neck and that the origin of the disease is poor diet and atmospheric influences.[24] His work published in 1762 by his friend Juan Sevillano was titled ‘Historia Natural y Medicina del Principado de Asturias’ or Natural and Medical History of the Principality of Asturias (1762). This led to the disease being known as "Asturian leprosy", and it is recognized as the first modern pathological description of a syndrome.[25] It was an endemic disease in northern Italy, where it was named pelle agra (pelle = skin; agra = sour) by Francesco Frapolli of Milan.[26] With pellagra affecting over 100,000 people in Italy by the 1880s, debates raged as to how to classify the disease (as a form of scurvy, elephantiasis or as something new), and over its causation. In the 19th century Roussel started a campaign in France to restrict consumption of maize and eradicated the disease in France, but it remained endemic in many rural areas of Europe.[27] Because pellagra outbreaks occurred in regions where maize was a dominant food crop, the most convincing hypothesis during the late nineteenth century, as espoused by Cesare Lombroso, was that the maize either carried a toxic substance or was a carrier of disease.[28] Louis Sambon, an Anglo-Italian doctor working at the London School of Tropical Medicine, was convinced that pellagra was carried by an insect, along the lines of malaria. Later, the lack of pellagra outbreaks in Mesoamerica, where maize is a major food crop, led researchers to investigate processing techniques in that region.
Pellagra was studied mostly in Europe until the late 19th century when it became an epidemic especially in the southern United States.[29] In the early 1900s, pellagra reached epidemic proportions in the American South. Between 1906 and 1940 more than 3 million Americans were affected by pellagra with more than 100,000 deaths, yet the epidemic resolved itself right after dietary niacin fortification.[30] Pellagra deaths in South Carolina numbered 1,306 during the first ten months of 1915; 100,000 Southerners were affected in 1916. At this time, the scientific community held that pellagra was probably caused by a germ or some unknown toxin in corn.[30] The Spartanburg Pellagra Hospital in Spartanburg, South Carolina, was the nation's first facility dedicated to discovering the cause of pellagra. It was established in 1914 with a special congressional appropriation to the U.S. Public Health Service (PHS) and set up primarily for research. In 1915, Joseph Goldberger, assigned to study pellagra by the Surgeon General of the United States, showed it was linked to diet by observing the outbreaks of pellagra in orphanages and mental hospitals. Goldberger noted that children between the ages of six and 12 (but not older or younger children at the orphanages) and patients at the mental hospitals (but not doctors or nurses) were the ones who seemed most susceptible to pellagra.[31] Goldberger theorized that a lack of meat, milk, eggs, and legumes made those particular populations susceptible to pellagra. By modifying the diet served in these institutions with "a marked increase in the fresh animal and the leguminous protein foods," Goldberger was able to show that pellagra could be prevented.[31] By 1926, Goldberger established that a diet that included these foods, or a small amount of brewer's yeast,[32] prevented pellagra.
Goldberger experimented on 11 prisoners (one was dismissed because of prostatitis). Before the experiment, the prisoners were eating the prison fare fed to all inmates at Rankin Prison Farm in Mississippi.[33] Goldberger started feeding them a restricted diet of grits, syrup, mush, biscuits, cabbage, sweet potatoes, rice, collards, and coffee with sugar (no milk). Healthy white male volunteers were selected as the typical skin lesions were easier to see in Caucasians and this population was felt to be those least susceptible to the disease, and thus provide the strongest evidence that the disease was caused by a nutritional deficiency. Subjects experienced mild, but typical cognitive and gastrointestinal symptoms, and within five months of this cereal-based diet, six of the 11 subjects broke out in the skin lesions that are necessary for a definitive diagnosis of pellagra. The lesions appeared first on the scrotum.[34] Goldberger was not given the opportunity to experimentally reverse the effects of diet-induced pellagra as the prisoners were released shortly after the diagnoses of pellagra were confirmed.[33] In the 1920s he connected pellagra to the diet of rural areas with corn-based diets rather than infection, contrary to the common medical ideas of that time.[35][36] Despite all his efforts, few physicians took up his ideas due to necessity of social reform, especially in the land system of that time, which led to many avoidable deaths and stereotypes.[37] Goldberger is remembered as the "unsung hero of American clinical epidemiology".[38] However, he failed to identify a specific element whose absence caused pellagra.
In 1937, Conrad Elvehjem, a biochemistry professor at the University of Wisconsin-Madison, showed that the vitamin niacin cured pellagra (manifested as black tongue) in dogs. Later studies by Dr. Tom Spies, Marion Blankenhorn, and Clark Cooper established that niacin also cured pellagra in humans, for which Time Magazine dubbed them its 1938 Men of the Year in comprehensive science.[39]
Research conducted between 1900 and 1950 found the number of cases of women with pellagra was consistently double the number of cases of afflicted men.[40] This is thought to be due to the inhibitory effect of estrogen on the conversion of the amino acid tryptophan to niacin.[41] Some researchers of the time gave a few explanations regarding the difference.[42]
Gillman and Gillman related skeletal tissue and pellagra in their research in South African Blacks. They provide some of the best evidence for skeletal manifestations of pellagra and the reaction of bone in malnutrition. They claimed radiological studies of adult pellagrins demonstrated marked osteoporosis. A negative mineral balance in pellagrins was noted, which indicated active mobilization and excretion of endogenous mineral substances, and undoubtedly impacted the turnover of bone. Extensive dental caries were present in over half of pellagra patients. In most cases, caries were associated with "severe gingival retraction, sepsis, exposure of cementum, and loosening of teeth".[43]
United States
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
|
8.8 g | |
| Tryptophan | 0.062 g |
| Vitamins | |
| Niacin (B3) |
(8%) 1.2 mg |
| |
| Percentages are roughly approximated using US recommendations for adults. | |
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
|
25 g | |
| Tryptophan | 0.2445 g |
| Vitamins | |
| Niacin (B3) |
(86%) 12.9 mg |
| |
| Percentages are roughly approximated using US recommendations for adults. | |
The whole dried corn kernel contains a nutritious germ and a thin seed coat that provides some fiber. [44] There are two important considerations for using ground whole-grain corn.
- The germ contains oil that is exposed by grinding, thus whole-grain cornmeal and grits turn rancid quickly at room temperature and should be refrigerated.
- Whole-grain cornmeal and grits require extended cooking times as seen in the following cooking directions for whole-grain grits;
"Place the grits in a pan and cover them with water. Allow the grits to settle a full minute, tilt the pan, and skim off and discard the chaff and hulls with a fine tea strainer. Cook the grits for 50 minutes if the grits were soaked overnight or else 90 minutes if not."[45]
Most of the niacin in mature cereal grains is present as niacytin, which is niacin bound up in a complex with hemicellulose which is nutritionally unavailable. In mature corn this may be up to 90% of the total niacin content.[46] The preparation method of nixtamalization using the whole dried corn kernel made this niacin nutritionally available and reduced the chance of developing pellagra. Niacytin is concentrated in the aleurone and germ layers which are removed by milling. The milling and degerming of corn in the preparation of cornmeal became feasible with the development of the Beall degerminator which was originally patented in 1901 and was used to separate the grit from the germ in corn processing.[47] However this process of degermination reduces the niacin content of the cornmeal.
Casimir Funk, who helped elucidate the role of thiamin in the etiology of beriberi, was an early investigator of the problem of pellagra. Funk suggested that a change in the method of milling corn was responsible for the outbreak of pellagra,[48] but no attention was paid to his article on this subject.[49]
Pellagra developed especially among the vulnerable populations in institutions such as orphanages and prisons, because of the monotonous and restricted diet. Soon pellagra began to occur in epidemic proportions in states south of the Potomac and Ohio rivers. The pellagra epidemic lasted for nearly four decades beginning in 1906.[49] It was estimated that there were 3 million cases and 100,000 deaths due to pellagra during the epidemic.[50]
See also
- Central chromatolysis
- Hartnup disease
- Harriette Chick
- Unethical human experimentation in the United States
References
- 1 2 Pitche P (2005). "Pellagra". Sante. 15 (3): 205–8. PMID 16207585.
- 1 2 Bapurao S, Krishnaswamy K (1978). "Vitamin B6 nutritional status of pellagrins and their leucine tolerance". Am J Clin Nutr. 31 (5): 819–24. PMID 206127.
- ↑ "Lysine | amino acid health benefits, dietary sources, side effects". Vitamins-supplements.org. Retrieved 2012-07-31.
- ↑ Hegyi, J.; Schwartz, R. A.; Hegyi, V. (2004). "Pellagra: Dermatitis, dementia, and diarrhea". International Journal of Dermatology. 43 (1): 1–5. doi:10.1111/j.1365-4632.2004.01959.x. PMID 14693013.
- ↑ Cleary MJ, Cleary JP (1989). "Anorexia nervosa: a form of subclinical pellagra". Int Clin Nutr Rev. 9 (3): 137–143. ISSN 0813-9008.
- ↑ FROSTIG, J. P., and SPIES, T. D.: The initial syndrome of pellagra and associated deficiency diseases. American Journal of the Medical Sciences 199:268, 1940.
- ↑ Gehring, W (2004). "Nicotinic acid/niacinamide and the skin". Journal of Cosmetic Dermatology. 3 (2): 88–93. doi:10.1111/j.1473-2130.2004.00115.x. PMID 17147561.
- ↑ Haas EM. "Vitamin B3—Niacin". Excepted from: Staying Healthy with Nutrition: The Complete Guide to Diet and Nutritional Medicine. Retrieved 2007-06-18.
- 1 2 3 4 Weise Prinzo, Z (2000). "Pellagra and its prevention and control in major emergencies" (pdf). World Health Organization. p. 24. WHO/NHD 00.10.
- ↑ {{url = http://usmle.biochemistryformedics.com/answer-case-study-pellagra-a-56-year-old-male-on-isoniazid-therapy-for-tuberculosis/}}
- ↑ Spark, Arlene (2007). Nutrition in Public Health: Principles, Policies, and Practice. CRC Press. p. 79. ISBN 9780203507889.
- ↑ Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A (2007). "Pellagra: a rare complication of anorexia nervosa". Eur Child Adolesc Psychiatry. 16 (7): 417–20. doi:10.1007/s00787-007-0613-4. PMID 17712518.
- ↑ Baquet, S.; Wuillaume, F.; van Egmond, K.; Ibañez, F. (2000). "Pellagra outbreak in Kuito, Angola". The Lancet. 355 (9217): 1829–1830. doi:10.1016/S0140-6736(05)73093-2.
- ↑ Dhakak, M; Limbu, B; Neopane, A; Karki, DB (2003). "A typical case of pellagra". Kathmandu University Medical Journal. 1 (1): 36–7. PMID 16340260.
- 1 2 3 Seal, AJ; Creeke, PI; Dibari, F; Cheung, E; Kyroussis, E; Semedo, P; van den Briel, T (2007). "Low and deficient niacin status and pellagra are endemic in postwar Angola". The American Journal of Clinical Nutrition. 85 (1): 218–24. PMID 17209199.
- ↑ Hegyi, J; Schwartz, RA; Hegyi, V (2004). "Pellagra: Dermatitis, dementia, and diarrhea". International Journal of Dermatology. 43 (1): 1–5. doi:10.1111/j.1365-4632.2004.01959.x. PMID 14693013.
- ↑ Prousky, JE (2003). "Pellagra may be a rare secondary complication of anorexia nervosa: A systematic review of the literature" (PDF). Alternative Medicine Review. 8 (2): 180–5. PMID 12777163.
- ↑ Pitsavas, S; Andreou, C; Bascialla, F; Bozikas, VP; Karavatos, A (2004). "Pellagra encephalopathy following B-complex vitamin treatment without niacin". International Journal of Psychiatry in Medicine. 34 (1): 91–5. doi:10.2190/29XV-1GG1-U17K-RGJH. PMID 15242145.
- ↑ Monteiro JP, da Cunha DF, Filho DC, Silva-Vergara ML, dos Santos VM, da Costa JC Jr., Etchebehere RM, Gonçalves J, de Carvalho da Cunha SF; et al. (2004). "Niacin metabolite excretion in alcoholic pellagra and AIDS patients with and without diarrhea". Nutrition. 20 (9): 778–82. doi:10.1016/j.nut.2004.05.008. PMID 15325687.
- ↑ Beretich, G.R. (2005). "Do high leucine/low tryptophan dieting foods (yogurt, gelatin) with niacin supplementation cause neuropsychiatric symptoms (depression) but not dermatological symptoms of pellagra?". Medical Hypotheses. 65 (3): 628–9. doi:10.1016/j.mehy.2005.04.002. PMID 15913906.
- ↑ Oliveira, A.; Sanches, M.; Selores, M. (2011). "Azathioprine-induced pellagra". The Journal of Dermatology. 38 (10): 1035–7. doi:10.1111/j.1346-8138.2010.01189.x. PMID 21658113.
- ↑ Delgado-Sanchez, L.; Godkar, D.; Niranjan, S. (2008). "Pellagra: Rekindling of an Old Flame". American Journal of Therapeutics. 15 (2): 173–5. doi:10.1097/MJT.0b013e31815ae309. PMID 18356638.
- ↑ Rajakumar, K (2000). "Pellagra in the United States: A Historical Perspective". Southern Medical Journal. 93 (3): 272–277. doi:10.1097/00007611-200093030-00005. ISSN 0038-4348. PMID 10728513.
- ↑ Casal, G. (1945). "The natural and medical history of the principality of the Asturias". In Major, RH. Classic Descriptions of Disease (3rd ed.). Springfield: Charles C Thomas. pp. 607–12.
- ↑ Stratigos, J.D.; Katsambas, A. (1977). "Pellagra: A still existing disease". British Journal of Dermatology. 96 (1): 99–106. doi:10.1111/j.1365-2133.1977.tb05197.x. PMID 843444.
- ↑ "Definition of Pellagra". MedicineNet.com. Retrieved 2007-06-18.
- ↑ Semba, RD (2000). "Théophile Roussel and the elimination of pellagra from 19th century France". Nutrition. 16 (3): 231–3. doi:10.1016/S0899-9007(99)00273-7. PMID 10705082.
- ↑ Cesare Lombroso, Studi clinici ed esperimentali sulla natura, causa e terapia delle pellagra (Bologna: Fava e Garagnani, 1869)
- ↑ Sydenstricker, VP (1958). "The history of pellagra, its recognition as a disorder of nutrition and its conquest". The American Journal of Clinical Nutrition. 6 (4): 409–14. PMID 13559167.
- 1 2 Bollet, AJ (1992). "Politics and pellagra: The epidemic of pellagra in the U.S. In the early twentieth century". The Yale Journal of Biology and Medicine. 65 (3): 211–21. PMC 2589605
 . PMID 1285449.
. PMID 1285449. - 1 2 "The Prevention of Pellagra: A Test of Diet among Institutional Inmates". JSTOR 4572932.
- ↑ Swan, P. (2005). "Goldberger's War: The Life and Work of a Public Health Crusader (review)". Bulletin of the History of Medicine. 79 (1): 146–7. doi:10.1353/bhm.2005.0046.
- 1 2 "Prisoners and Pellagra" (PDF).
- ↑ "Experimental Pellagra in the Human Subject Brought about by a Restricted Diet". JSTOR 4572984.
- ↑ Goldberger, J; Wheeler, GA (Nov 12, 1915). "Experimental pellagra in the human subject brought about by a restricted diet.". Public Health Reports. 30 (46): 3336–3339. doi:10.2307/4572984. JSTOR 4572984.
- ↑ Goldberger, J (2006). "The etiology of pellagra. 1914". Public Health Reports. 121 (Suppl 1): 77–9; discussion 76. PMID 16550768.
- ↑ Wolf, R; Orion, E; Matz, H; Tüzün, Y; Tüzün, B (2002). "Miscellaneous treatments, II: Niacin and heparin: Unapproved uses, dosages, or indications". Clinics in Dermatology. 20 (5): 547–57. doi:10.1016/S0738-081X(02)00268-7. PMID 12435525.
- ↑ Elmore, JG; Feinstein, AR (1994). "Joseph Goldberger: An unsung hero of American clinical epidemiology". Annals of Internal Medicine. 121 (5): 372–5. doi:10.7326/0003-4819-121-5-199409010-00010. PMID 8042827.
- ↑ Ruth Hanna Sachs, White Rose History. Volume I. 2003. Appendix D, p.2 ISBN 0971054193 "Men of the Year, outstanding in comprehensive science were three medical researchers who discovered that nicotinic acid was a cure for human pellagra: Drs. Tom Douglas Spies of Cincinnati General Hospital, Marion Arthur Blankenhorn of the University of Cincinnati, Clark Niel Cooper of Waterloo, Iowa."
- ↑ Miller DF (1978). "Pellagra deaths in the United States". Am. J. Clin. Nutr. 31 (4): 558–9. PMID 637029.
- ↑ Brenton, B. P. (2000). "Pellagra, Sex and Gender: Biocultural Perspectives on Differential Diets and Health". Nutritional Anthropology. 23 (1): 20–4. doi:10.1525/nua.2000.23.1.20.
- ↑ Carpenter, K. (1981). Pellagra. Stroudsburg, PA: Hutchinson Ross Pub. Co. ISBN 0-87933-364-2.
- ↑ Gillman, J.; Gillman, T. (1951). Perspectives in Human Malnutrition: A Contribution to the Biology of Disease from a Clinical and Pathological Study of Chronic Malnutrition and Pellagra in the African. New York, NY: Grune and Stratton.
- ↑ Fletcher, Janet. "WAVES OF GRAIN / Grain glossary". SFGate. Hearst. Retrieved 2 October 2014.
- ↑ "Simple Buttered Antebellum Coarse Grits". Anson Mills. Anson Mills.
- ↑ Ball, George F.M. (2005). Vitamins In Foods: Analysis, Bioavailability, and Stability; Food Science and Technology. CRC Press. p. 183. ISBN 9781420026979. Retrieved 12 October 2014.
- ↑ "Beall Degerminators General Information". Beall Degerminators. Beall Degerminator Company. Retrieved 2 October 2014.
- ↑ Funk C (1913). "Studies on pellagra. The influence of the milling of maize on the chemical composition and nutritive value of the meal". J Physiol. 47: 389–392. doi:10.1113/jphysiol.1913.sp001631.
- 1 2 Alfred JAY Bollet (1992). "Politics and Pellagra: The Epidemic of Pellagra in the U.S. in the Early Twentieth Century. (PDF)" (PDF). The Yale Journal of Biology and Medicine. 65: 211–221. PMC 2589605
 . PMID 1285449.
. PMID 1285449. - ↑ KUMARAVEL, RAJAKUMAR. "Pellagra in the United States: A Historical Perspective" (PDF). Solving Practical Problems with Mathematics. John F. McGowan, Ph.D. Retrieved 2 October 2014.
Further reading
- Hampl JS, Hampl WS (1 November 1997). "Pellagra and the origin of a myth: evidence from European literature and folklore". Journal of the Royal Society of Medicine. 90 (11): 636–9. PMC 1296679
 . PMID 9496281.
. PMID 9496281. - "Reports and Resolutions of the General Assembly of the State of South Carolina, Regular Session Commencing January 11, 1916". Annual Report of the State Board of Health (1915-1916). Columbia, S.C.: Gonzales and Bryan, state printers. 4. 1916.
- Beardsley E (2006). The Spartanburg Pellagra Hospital. In: The South Carolina Encyclopedia. Columbia, S.C: University of South Carolina Press. ISBN 1-57003-598-9.
- Swain CP, Tavill AS, Neale G (September 1976). "Studies of tryptophan and albumin metabolism in a patient with carcinoid syndrome, pellagra, and hypoproteinemia". Gastroenterology. 71 (3): 484–9. PMID 133045.
- Hendrick, Burton J. (April 1916). "The Mastery Of Pellagra: The Mysterious Disease, Almost Unknown In This Country Fifteen Years Ago, That Now Claims 7,500 Victims A Year And Is Spreading Rapidly". The World's Work: A History of Our Time. XXXI: 633–639. Retrieved 2009-08-04.
- Kraut, Alan. "Dr. Joseph Goldberger and the War on Pellagra, By Alan Kraut, Ph.D." Office of History, National Institutes of Health. Web. 03 Sept. 2010.
