Terminal deoxynucleotidyl transferase
| DNTT | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||
| |||||||||||||||||
| Identifiers | |||||||||||||||||
| Aliases | DNTT, TDT, DNA nucleotidylexotransferase, Terminal deoxynucleotidyl transferase | ||||||||||||||||
| External IDs | OMIM: 187410 MGI: 98659 HomoloGene: 3014 GeneCards: DNTT | ||||||||||||||||
| |||||||||||||||||
| Orthologs | |||||||||||||||||
| Species | Human | Mouse | |||||||||||||||
| Entrez | |||||||||||||||||
| Ensembl | |||||||||||||||||
| UniProt | |||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||
| RefSeq (protein) | |||||||||||||||||
| Location (UCSC) | Chr 10: 96.3 – 96.34 Mb | Chr 19: 41.03 – 41.06 Mb | |||||||||||||||
| PubMed search | [1] | [2] | |||||||||||||||
| Wikidata | |||||||||||||||||
| View/Edit Human | View/Edit Mouse |
Terminal deoxynucleotidyl transferase (TdT), also known as DNA nucleotidylexotransferase (DNTT) or terminal transferase, is a specialized DNA polymerase expressed in immature, pre-B, pre-T lymphoid cells, and acute lymphoblastic leukemia/lymphoma cells. TdT adds N-nucleotides to the V,D, and J exons of the TCR and BCR genes during antibody gene recombination, enabling the phenomenon of junctional diversity. In humans, terminal transferase is encoded by the DNTT gene.[3][4] As a member of the X family of DNA polymerase enzymes, it works in conjunction with polymerase λ and polymerase μ, both of which belong to the same X family of polymerase enzymes. The diversity introduced by TdT has played an important role in the evolution of the vertebrate immune system, significantly increasing the variety of antigen receptors that a cell is equipped with to fight pathogens. Studies using TdT knockout mice have found drastic reductions (10-fold) in T-Cell receptor (TCR) diversity compared with that of normal, or wild-type, systems. The greater diversity of TCRs that an organism is equipped with leads to greater resistance to infection.[5][6]
TdT is absent in fetal liver HSCs, significantly impairing junctional diversity in B-cells during the fetal period.[7]
Function and regulation
TdT catalyses the addition of nucleotides to the 3' terminus of a DNA molecule. Unlike most DNA polymerases, it does not require a template. The preferred substrate of this enzyme is a 3'-overhang, but it can also add nucleotides to blunt or recessed 3' ends. Cobalt is a necessary cofactor, however the enzyme catalyzes reaction upon Mg and Mn administration in vitro.
TdT is expressed mostly in the primary lymphoid organs, like the thymus and bone marrow. Regulation of its expression occurs via multiple pathways. These include protein-protein interactions, like those with TdIF1. TdIF1 is another protein that interacts with TdT to inhibit its function by masking the DNA binding region of the TdT polymerase. The regulation of TdT expression also exists at the transcriptional level, with regulation influenced by stage-specific factors, and occurs in a developmentally restrictive manner.[5][8][9] Although expression is typically found to be in the primary lymphoid organs, recent work has suggested that stimulation via antigen can result in secondary TdT expression along with other enzymes needed for gene rearrangement outside of the thymus for T-cells.[10]
Mechanism
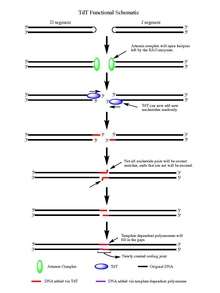
Upon the action of the RAG 1/2 enzymes, the cleaved double stranded DNA is left with hairpin structures at the end of each DNA segment created by the cleavage event. The hairpins are both opened by the Artemis complex, which has endonuclease activity when phosphorylated, providing the free 3' OH ends for TdT to act upon. Once the Artemis complex has done its job and added palindromic nucleotides (P-nucleotides) to the newly opened DNA hairpins, the stage is set for TdT to do its job. TdT is now able to come in and add N-nucleotides to the existing P-nucleotides in a 5' to 3' direction that polymerases are known to function. On average 2-5 random base pairs are added to each 3' end generated after the action of the Artemis complex. The number of bases added is enough for the two newly synthesized ssDNA segments to undergo micro homology alignment during non-homologous end joining according to the normal Watson-Crick base pairing patterns (A-T, C-G). From there unpaired nucleotides are excised by an exonuclease, like the Artemis Complex (which has exonuclease activity in addition to endonuclease activity), and then template dependent polymerases are able to fill the gaps, finally creating the new coding joint with the action of ligase to combine the segments. Although TdT does not discriminate among the four base pairs when adding them to the N-nucleotide segments, it has shown a bias for guanine and cytosine base pairs.[5]
Polymerase λ has also been found to exhibit similar template independent synthetic activity. Along with activity as a terminal transferase, it is known to also work in a template dependent fashion. This is interesting because TdT works exclusively via a template independent pathway for the addition of nucleotides.[11]
Uses
Terminal transferase has applications in molecular biology. It can be used in RACE to add nucleotides that can then be used as a template for a primer in subsequent PCR. It can also be used to add nucleotides labeled with radioactive isotopes, for example in the TUNEL assay (Terminal deoxynucleotidyl transferase dUTP Nick End Labeling) for the demonstration of apoptosis (which is marked, in part, by fragmented DNA). It is also used in the immunofluorescence assay for the diagnosis of acute lymphoblastic leukemia.[12]
In immunohistochemistry, antibodies to TdT can be used to demonstrate the presence of immature T and B cells and pluripotent hematopoietic stem cells, which possess the antigen, while mature lymphoid cells are always TdT-negative. While TdT-positive cells are found in small numbers in healthy lymph nodes and tonsils, the malignant cells of acute lymphoblastic leukaemia are also TdT-positive, and the antibody can, therefore, be used as part of a panel to diagnose this disease and to distinguish it from, for example, small cell tumours of childhood.[13]
See also
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Isobe M, Huebner K, Erikson J, Peterson RC, Bollum FJ, Chang LM, Croce CM (Sep 1985). "Chromosome localization of the gene for human terminal deoxynucleotidyltransferase to region 10q23-q25". Proceedings of the National Academy of Sciences of the United States of America. 82 (17): 5836–40. doi:10.1073/pnas.82.17.5836. PMC 390648
 . PMID 3862101.
. PMID 3862101. - ↑ Yang-Feng TL, Landau NR, Baltimore D, Francke U (1986). "The terminal deoxynucleotidyltransferase gene is located on human chromosome 10 (10q23----q24) and on mouse chromosome 19". Cytogenetics and Cell Genetics. 43 (3-4): 121–6. doi:10.1159/000132309. PMID 3467897.
- 1 2 3 Motea EA, Berdis AJ (May 2010). "Terminal deoxynucleotidyl transferase: the story of a misguided DNA polymerase". Biochimica et Biophysica Acta. 1804 (5): 1151–66. doi:10.1016/j.bbapap.2009.06.030. PMC 2846215
 . PMID 19596089.
. PMID 19596089. - ↑ Haeryfar SM, Hickman HD, Irvine KR, Tscharke DC, Bennink JR, Yewdell JW (Jul 2008). "Terminal deoxynucleotidyl transferase establishes and broadens antiviral CD8+ T cell immunodominance hierarchies". Journal of Immunology. 181 (1): 649–59. doi:10.4049/jimmunol.181.1.649. PMC 2587314
 . PMID 18566432.
. PMID 18566432. - ↑ Hardy R (2008). "Chapter 7: B Lymphocyte Development and Biology". In Paul W. Fundamental Immunology (Book) (6th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 237–269. ISBN 0-7817-6519-6.
- ↑ Cherrier M, D'Andon MF, Rougeon F, Doyen N (Feb 2008). "Identification of a new cis-regulatory element of the terminal deoxynucleotidyl transferase gene in the 5' region of the murine locus". Molecular Immunology. 45 (4): 1009–17. doi:10.1016/j.molimm.2007.07.027. PMID 17854898.
- ↑ Kubota T, Maezawa S, Koiwai K, Hayano T, Koiwai O (Aug 2007). "Identification of functional domains in TdIF1 and its inhibitory mechanism for TdT activity". Genes to Cells. 12 (8): 941–59. doi:10.1111/j.1365-2443.2007.01105.x. PMID 17663723.
- ↑ Zhang Y, Shi M, Wen Q, Luo W, Yang Z, Zhou M, Ma L (2012-01-01). "Antigenic stimulation induces recombination activating gene 1 and terminal deoxynucleotidyl transferase expression in a murine T-cell hybridoma". Cellular Immunology. 274 (1-2): 19–25. doi:10.1016/j.cellimm.2012.02.008. PMID 22464913.
- ↑ Maga G, Ramadan K, Locatelli GA, Shevelev I, Spadari S, Hübscher U (Jan 2005). "DNA elongation by the human DNA polymerase lambda polymerase and terminal transferase activities are differentially coordinated by proliferating cell nuclear antigen and replication protein A". The Journal of Biological Chemistry. 280 (3): 1971–81. doi:10.1074/jbc.M411650200. PMID 15537631.
- ↑ Faber J, Kantarjian H, Roberts MW, Keating M, Freireich E, Albitar M (Jan 2000). "Terminal deoxynucleotidyl transferase-negative acute lymphoblastic leukemia". Archives of Pathology & Laboratory Medicine. 124 (1): 92–7. doi:10.1043/0003-9985(2000)124<0092:TDTNAL>2.0.CO;2. PMID 10629138.
- ↑ Leong, Anthony S-Y; Cooper, Kumarason; Leong, F Joel W-M (2003). Manual of Diagnostic Cytology (2 ed.). Greenwich Medical Media, Ltd. pp. 413–414. ISBN 1-84110-100-1.
Further reading
- O'Malley DP, Orazi A (Aug 2006). "Terminal deoxynucleotidyl transferase-positive cells in spleen, appendix and branchial cleft cysts in pediatric patients". Haematologica. 91 (8): 1139–40. PMID 16885057.
- Yamashita N, Shimazaki N, Ibe S, Kaneko R, Tanabe A, Toyomoto T, Fujita K, Hasegawa T, Toji S, Tamai K, Yamamoto H, Koiwai O (Jul 2001). "Terminal deoxynucleotidyltransferase directly interacts with a novel nuclear protein that is homologous to p65". Genes to Cells. 6 (7): 641–52. doi:10.1046/j.1365-2443.2001.00449.x. PMID 11473582.
- Chang LM, Bollum FJ (1986). "Molecular biology of terminal transferase". CRC Critical Reviews in Biochemistry. 21 (1): 27–52. doi:10.3109/10409238609113608. PMID 3524991.
- Maezawa S, Hayano T, Koiwai K, Fukushima R, Kouda K, Kubota T, Koiwai O (May 2008). "Bood POZ containing gene type 2 is a human counterpart of yeast Btb3p and promotes the degradation of terminal deoxynucleotidyltransferase". Genes to Cells. 13 (5): 439–57. doi:10.1111/j.1365-2443.2008.01179.x. PMID 18429817.
- Taplin ME, Frantz ME, Canning C, Ritz J, Blumberg RS, Balk SP (Mar 1996). "Evidence against T-cell development in the adult human intestinal mucosa based upon lack of terminal deoxynucleotidyltransferase expression". Immunology. 87 (3): 402–7. doi:10.1046/j.1365-2567.1996.496571.x. PMC 1384108
 . PMID 8778025.
. PMID 8778025. - Grupe A, Li Y, Rowland C, Nowotny P, Hinrichs AL, Smemo S, Kauwe JS, Maxwell TJ, Cherny S, Doil L, Tacey K, van Luchene R, Myers A, Wavrant-De Vrièze F, Kaleem M, Hollingworth P, Jehu L, Foy C, Archer N, Hamilton G, Holmans P, Morris CM, Catanese J, Sninsky J, White TJ, Powell J, Hardy J, O'Donovan M, Lovestone S, Jones L, Morris JC, Thal L, Owen M, Williams J, Goate A (Jan 2006). "A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease". American Journal of Human Genetics. 78 (1): 78–88. doi:10.1086/498851. PMC 1380225
 . PMID 16385451.
. PMID 16385451. - Dworzak MN, Fritsch G, Fröschl G, Printz D, Gadner H (Nov 1998). "Four-color flow cytometric investigation of terminal deoxynucleotidyl transferase-positive lymphoid precursors in pediatric bone marrow: CD79a expression precedes CD19 in early B-cell ontogeny". Blood. 92 (9): 3203–9. PMID 9787156.
- Fujita K, Shimazaki N, Ohta Y, Kubota T, Ibe S, Toji S, Tamai K, Fujisaki S, Hayano T, Koiwai O (Jun 2003). "Terminal deoxynucleotidyltransferase forms a ternary complex with a novel chromatin remodeling protein with 82 kDa and core histone". Genes to Cells. 8 (6): 559–71. doi:10.1046/j.1365-2443.2003.00656.x. PMID 12786946.
- Kubota T, Maezawa S, Koiwai K, Hayano T, Koiwai O (Aug 2007). "Identification of functional domains in TdIF1 and its inhibitory mechanism for TdT activity". Genes to Cells. 12 (8): 941–59. doi:10.1111/j.1365-2443.2007.01105.x. PMID 17663723.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1-2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Bridges SL (Aug 1998). "Frequent N addition and clonal relatedness among immunoglobulin lambda light chains expressed in rheumatoid arthritis synovia and PBL, and the influence of V lambda gene segment utilization on CDR3 length". Molecular Medicine. 4 (8): 525–53. PMC 2230400
 . PMID 9742508.
. PMID 9742508. - Liu L, McGavran L, Lovell MA, Wei Q, Jamieson BA, Williams SA, Dirks NN, Danielson MS, Dubie LM, Liang X (Jun 2004). "Nonpositive terminal deoxynucleotidyl transferase in pediatric precursor B-lymphoblastic leukemia". American Journal of Clinical Pathology. 121 (6): 810–5. doi:10.1309/QD18-PPV1-NH3T-EUTF. PMID 15198352.
- Yang B, Gathy KN, Coleman MS (Apr 1994). "Mutational analysis of residues in the nucleotide binding domain of human terminal deoxynucleotidyl transferase". The Journal of Biological Chemistry. 269 (16): 11859–68. PMID 8163485.
- Thai TH, Kearney JF (Sep 2004). "Distinct and opposite activities of human terminal deoxynucleotidyltransferase splice variants". Journal of Immunology. 173 (6): 4009–19. doi:10.4049/jimmunol.173.6.4009. PMID 15356150.
- Shimazaki N, Fujita K, Koiwai O (Mar 2002). "[Expression and function of terminal deoxynucleotidyl-transferase and discovery of novel DNA polymerase mu]". Seikagaku. The Journal of Japanese Biochemical Society. 74 (3): 227–32. PMID 11974916.
- Mahajan KN, Mitchell BS (Sep 2003). "Role of human Pso4 in mammalian DNA repair and association with terminal deoxynucleotidyl transferase". Proceedings of the National Academy of Sciences of the United States of America. 100 (19): 10746–51. doi:10.1073/pnas.1631060100. PMC 196874
 . PMID 12960389.
. PMID 12960389. - Mahajan KN, Gangi-Peterson L, Sorscher DH, Wang J, Gathy KN, Mahajan NP, Reeves WH, Mitchell BS (Nov 1999). "Association of terminal deoxynucleotidyl transferase with Ku". Proceedings of the National Academy of Sciences of the United States of America. 96 (24): 13926–31. doi:10.1073/pnas.96.24.13926. PMC 24167
 . PMID 10570175.
. PMID 10570175. - Ibe S, Fujita K, Toyomoto T, Shimazaki N, Kaneko R, Tanabe A, Takebe I, Kuroda S, Kobayashi T, Toji S, Tamai K, Yamamoto H, Koiwai O (Sep 2001). "Terminal deoxynucleotidyltransferase is negatively regulated by direct interaction with proliferating cell nuclear antigen". Genes to Cells. 6 (9): 815–24. doi:10.1046/j.1365-2443.2001.00460.x. PMID 11554927.
External links
- Terminal Deoxyribonucleotidyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)
