Amphotericin B
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fungizone, Mysteclin-F |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | I.V. (slow infusion only) topical |
| ATC code | A01AB04 (WHO) A07AA07 (WHO), G01AA03 (WHO), J02AA01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | renal |
| Biological half-life |
initial phase : 24 hours, second phase : approx. 15 days |
| Excretion |
40% found in urine after single cumulated over several days biliar excretion also important |
| Identifiers | |
| |
| CAS Number |
1397-89-3 |
| PubChem (CID) | 14956 |
| DrugBank |
DB00681 |
| ChemSpider |
10237579 |
| KEGG |
D00203 |
| ChEBI |
CHEBI:2682 |
| ChEMBL |
CHEMBL267345 |
| NIAID ChemDB | 000096 |
| Chemical and physical data | |
| Formula | C47H73NO17 |
| Molar mass | 924.091 |
| 3D model (Jmol) | Interactive image |
| Melting point | 170 °C (338 °F) |
| |
| |
| (verify) | |
Amphotericin B is an antifungal drug often used intravenously for serious systemic fungal infections and is the only effective treatment for some fungal infections.[1]
Common side effects include a reaction of fever, shaking chills, headaches and low blood pressure soon after it is infused, as well as kidney and electrolyte problems.[1] Allergic symptoms including anaphylaxis may occur.[1]
It is of the polyene class. It is a subgroup of the macrolide antibiotics, and exhibits similar structural elements.[2] Currently, the drug is available in many forms. Either "conventionally" complexed with sodium deoxycholate (ABD), as a cholesteryl sulfate complex (ABCD), as a lipid complex (ABLC), and as a liposomal formulation (LAMB). The latter formulations have been developed to improve tolerability and decrease toxicity, but may show considerably different pharmacokinetic characteristics compared to conventional amphotericin B.[3]
It was originally made from Streptomyces nodosus in 1955.[4] Its name originates from the chemical's amphoteric properties. It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[5]
Medical uses
Antifungal
One of the main uses of amphotericin B is treating a wide range of systemic fungal infections. Due to its extensive side effects, it is often reserved for severe infections in critically ill, or immunocompromised patients. It is considered first line therapy for invasive mucormycosis infections, cryptococcal meningitis, and certain aspergillus and candidal infections.[6][7] It has been a highly effective drug for over fifty years in large part because it has a low incidence of drug resistance in the pathogens it treats. This is because amphotericin B resistance requires sacrifices on the part of the pathogen that make it susceptible to the host environment, and too weak to cause infection.[8]
Antiprotozoal
Amphotericin B is often used in otherwise-untreatable protozoan infections such as visceral leishmaniasis[9] and primary amoebic meningoencephalitis.[10]
Spectrum of susceptibility
The following table shows the amphotericin B susceptibility for a selection of medically important fungi.
| Species | Amphotericin B
MIC breakpoint (mg/L) |
|---|---|
| Aspergillus fumigatus | 1[11] |
| Aspergillus terreus | Resistant[3][11] |
| Candida albicans | 1[11] |
| Candida krusei | 1[11] |
| Candida glabrata | 1[11] |
| Candida lusitaniae | Intrinsically resistant[3] |
| Cryptococcus neoformans | 2[12] |
| Fusarium oxysporum | 2[12] |
Route of administration
Intravenous
Deoxycholate
Amphotericin B alone is insoluble in normal saline at a pH of 7. The original formulation used sodium deoxycholate to improve solubility.[3] Amphotericin B deoxycholate (ABD) is administered IV, though with frequent adverse effects as detailed in the side effects section.[13] As the original formulation of amphotericin, it is often referred to as "conventional" amphotericin.[14]
Liposomal formulations
In order to improve the tolerability of amphotericin and reduce toxicity, several lipid formulations have been developed.[3]
From studies, it appears that liposomal amphotericin B preparations exhibit fewer side effects, while having similar efficacy. Various preparations have recently been introduced. All of these are more expensive than plain amphotericin B.
AmBisome (LAMB) is a liposomal formulation of amphotericin B for injection, developed by NeXstar Pharmaceuticals (acquired by Gilead Sciences in 1999). It was approved by the FDA in 1997.[15] It is marketed by Gilead in Europe and licensed to Astellas Pharma (formerly Fujisawa Pharmaceuticals) for marketing in the USA, and Sumitomo Pharmaceuticals in Japan. It consists of a mixture of phosphatidylcholine, cholesterol and distearoyl phosphatidylglycerol that in aqueous media they spontaneously arrange into unilamellar vesicles that contain amphotericin B.[3][16]
Liposomal formulations have been primarily used as it has been found to have less renal toxicity than deoxycholate.[17] Guidelines also recommend the use of LAMB for CNS fungal infections due to better pharmacokinetics and CNS penetration but do note that this is based only on animal models.[18][19]
Fungisome[20] is a generic liposomal complex of amphotericin B. It is marketed by Lifecare Innovations of India.
Lipid complex formulations
Amphotec (ABCD) and Abelcet (ABLC) are lipid complex preparations. Abelcet was approved by the FDA in 1995.[21] It consists of amphotericin B and two lipids in a 1:1 ratio that form large ribbon-like structures.[3] Amphotec is a complex of amphotericin and sodium cholesteryl sulfate in a 1:1 ratio. Two molecules of each form a tetramer that aggregate into spiral arms on a disk-like complex.[16] It was approved by the FDA in 1996.[21] Neither of these are true unilamellar liposomes like ambisome.
Oral preparations
A major barrier to the use of amphotericin in resource-poor settings is that it must be given intravenously (except for topical applications). An oral preparation exists, but is not commercially available.[22] The amphipathic nature of amphotericin along with its low solubility and permeability has posed major hurdles for oral administration given its low bioavailability. In the past it had been used for fungal infections of the surface of the GI tract such as thrush, but has been replaced by other antifungals such as nystatin and fluconazole.[23]
However, recently novel nanoparticulate drug delivery systems such as AmbiOnp,[24] nanosuspensions, lipid-based drug delivery systems including cochleates, self-emulsifying drug delivery systems,[25] solid lipid nanoparticles[26] and polymeric nanoparticles[27]—such as Amphotericin B in pegylated polylactide coglycolide copolymer nanoparticles[28]—have demonstrated potential for oral formulation of amphotericin B.[29]
Side effects
Amphotericin B is well known for its severe and potentially lethal side effects. Very often, a serious acute reaction after the infusion (1 to 3 hours later) is noted, consisting of high fever, shaking chills (leading to the medical slang term "shake and bake"),[30] hypotension, anorexia, nausea, vomiting, headache, dyspnea and tachypnea, drowsiness, and generalized weakness. This reaction sometimes subsides with later applications of the drug, and may in part be due to histamine liberation. An increase in prostaglandin synthesis may also play a role. This nearly universal febrile response necessitates a critical (and diagnostically difficult) professional determination as to whether the onset of high fever is a novel symptom of a fast-progressing disease, or merely the effect of the drug. To decrease the likelihood and severity of the symptoms, initial doses should be low, and increased slowly. Paracetamol, pethidine, diphenhydramine, and hydrocortisone have all been used to treat or prevent the syndrome, but the prophylactic use of these drugs is often limited by the patient's condition.
Intravenously administered amphotericin B in therapeutic doses has also been associated with multiple organ damage. Kidney damage is a frequently reported side effect, and can be severe and/or irreversible. Less kidney toxicity has been reported with liposomal formulations such as AmBisome) and it has become preferred in patients with preexisting renal injury.[31][32] The integrity of the liposome is disrupted when it binds to the fungal cell wall, but is not affected by the mammalian cell membrane,[33] so the association with liposomes decreases the exposure of the kidneys to amphotericin B, which explains its less nephrotoxic effects.[34]
In addition, electrolyte imbalances such as hypokalemia and hypomagnesemia are also common.[35] In the liver, increased liver enzymes and hepatotoxicity (up to and including fulminant liver failure) are common. In the circulatory system, several forms of anemia and other blood dyscrasias (leukopenia, thrombopenia), serious cardiac arrhythmias (including ventricular fibrillation), and even frank cardiac failure have been reported. Skin reactions, including serious forms, are also possible.
Interactions
- Flucytosine: Toxicity of flucytosine is increased and allows a lower dose of amphotericin B. Amphotericin B may also facilitate entry of flucystosine into the fungal cell by interfering with the permeability of the fungal cell membrane.
- Diuretics or cisplatin: Increased renal toxicity and increased risk of hypokalemia
- Corticosteroids: Increased risk of hypokalemia
- Cytostatic drugs: Increased risk of kidney damage, hypotension, and bronchospasms
- Other nephrotoxic drugs (such as aminoglycosides): Increased risk of serious renal damage
- Foscarnet, ganciclovir, tenofovir, adefovir: Risk of hematological and renal side effects of amphotericin B are increased
- Transfusion of leukocytes: Risk of pulmonal (lung) damage occurs, space the intervals between the application of amphotericin B and the transfusion, and monitor pulmonary function
Mechanism of action
Amphotericin B binds with ergosterol, a component of fungal cell membranes, forming pores that cause rapid leakage of monovalent ions (K+, Na+, H+ and Cl−) and subsequent fungal cell death. This is amphotericin B's primary effect as an antifungal agent.[36][37] It has been found that the amphotericin B/ergosterol bimolecular complex that maintains these pores is stabilized by Van der Waals interactions.[38] Researchers have found evidence that amphotericin B also causes oxidative stress within the fungal cell,[39] but it remains unclear to what extent this oxidative damage contributes to the drug's effectiveness.[36] The addition of free radical scavengers or antioxidants can lead to amphotericin resistance in some species, such as Scedosporium prolificans, without affecting the cell wall.
Two amphotericins, amphotericin A and amphotericin B, are known, but only B is used clinically, because it is significantly more active in vivo. Amphotericin A is almost identical to amphotericin B (having a double C=C bond between the 27th and 28th carbons), but has little antifungal activity.[40]
Mechanism of toxicity
Mammalian and fungal membranes both contain sterols, a primary membrane target for amphotericin B. Because mammalian and fungal membranes are similar in structure and composition, this is one mechanism by which amphotericin B causes cellular toxicity. Amphotericin B molecules can form pores in the host membrane as well as the fungal membrane. This impairment in membrane barrier function can have lethal effects.[39][41][42] Ergosterol, the fungal sterol, is more sensitive to amphotericin B than cholesterol, the common mammalian sterol. Reactivity with the membrane is also sterol concentration dependent.[43] Bacteria are not affected as their cell membranes do not contain sterols.
Amphotericin administration is limited by infusion-related toxicity. This is thought to result from innate immune production of proinflammatory cytokines.[41][44]
Biosynthesis
The natural route to synthesis includes polyketide synthase components.[45] The carbon chains of Amphotericin B are assembled from sixteen ‘C2’ acetate and three ‘C3’propionate units by polyketide synthases (PKSs).[46] Polyketide biosynthesis begins with the decarboxylative condensation of a dicarboxylic acid extender unit with a starter acyl unit to form a β-ketoacyl intermediate. The growing chain is constructed by a series of Claisen reactions. Within each module, the extender units are loaded onto the current ACP domain by acetyl transferase (AT). The ACP-bound elongation group reacts in a Claisen condensation with the KS-bound polyketide chain. Ketoreductase (KR), dehydratase (DH) and enoyl reductase (ER) enzymes may also be present to form alcohol, double bonds or single bonds.[47] After cyclisation, the macrolactone core undergoes further modification by hydroxylation, methylation and glycosylation. The order of these processes is unknown.
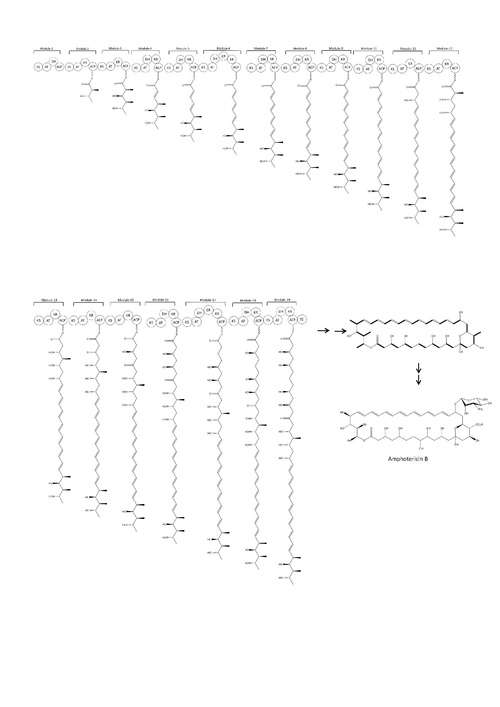
History
It was originally extracted from Streptomyces nodosus, a filamentous bacterium, in 1955, at the Squibb Institute for Medical Research from cultures of an undescribed streptomycete isolated from the soil collected in the Orinoco River region of Venezuela.[40] Two antifungal substances were isolated from the soil culture, Amphotericin A and Amphotericin B, but B had better antifungal activity. For decades it remained it the only effective therapy for invasive fungal disease until the development of the azole antifungals in the early 1980s.[13]
Its complete stereo structure was determined in 1970 by an X-ray structure of the N-iodoacetyl derivative.[48] The first synthesis of the compound's naturally occurring enantiomeric form was achieved in 1987.[49]
Society and culture
Brand names
Fungilin, Fungizone, Abelcet, AmBisome, Fungisome, Amphocil, Amphotec, Halizon[50]
References
- 1 2 3 "Amphotericin B". The American Society of Health-System Pharmacists. Retrieved Jan 1, 2015.
- ↑ "Chemistry and Biology of the Polyene Macrolide Antibiotics". Bacteriological Reviews. 32.
- 1 2 3 4 5 6 7 Hamill, Richard J. (2013-06-01). "Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity". Drugs. 73 (9): 919–934. doi:10.1007/s40265-013-0069-4. ISSN 0012-6667.
- ↑ Walker, S. R. (2012). Trends and Changes in Drug Research and Development. Springer Science & Business Media. p. 109. ISBN 9789400926592.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Drugs Active against Fungi, Pneumocystis, and Microsporidia. pp. 479–494.e4. ISBN 978-1-4557-4801-3.
- ↑ Moen, Marit D.; Lyseng-Williamson, Katherine A.; Scott, Lesley J. (2012-09-17). "Liposomal Amphotericin B". Drugs. 69 (3): 361–392. doi:10.2165/00003495-200969030-00010. ISSN 0012-6667. PMID 19275278.
- ↑ Rura, Nicole (2013-10-29). "Understanding the evolution of drug resistance points to novel strategy for developing better antimicrobials". Retrieved 2016-11-14 – via Whitehead Institute.
- ↑ den Boer, Margriet; Davidson, Robert N. (2006-04-01). "Treatment options for visceral leishmaniasis". Expert Review of Anti-Infective Therapy. 4 (2): 187–197. doi:10.1586/14787210.4.2.187. ISSN 1744-8336. PMID 16597201.
- ↑ Grace, Eddie; Asbill, Scott; Virga, Kris (November 2015). "Naegleria fowleri: Pathogenesis, Diagnosis, and Treatment Options". Antimicrobial Agents and Chemotherapy. 59 (11): 6677–6681. doi:10.1128/AAC.01293-15. PMC 4604384
 . PMID 26259797.
. PMID 26259797. - 1 2 3 4 5 "European Committee on Antimicrobial Susceptibility Testing: Antifungal Agents, Breakpoint tables for interpretation of MICs" (PDF). 2015-11-16. Retrieved 2015-11-17.
- 1 2 "Index | The Antimicrobial Index Knowledgebase - TOKU-E". antibiotics.toku-e.com. Retrieved 2015-11-17.
- 1 2 Maertens, J. A. (2004-03-01). "History of the development of azole derivatives". Clinical Microbiology and Infection. 10: 1–10. doi:10.1111/j.1470-9465.2004.00841.x. ISSN 1469-0691.
- ↑ Clemons, KV; Stevens, DA (April 1998). "Comparison of Fungizone, Amphotec, AmBisome, and Abelcet for Treatment of Systemic Murine Cryptococcosis". Antimicrobial Agents and Chemotherapy. 42: 899–902. PMC 105563
 . PMID 9559804.
. PMID 9559804. - ↑ "Drug Approval Package". www.accessdata.fda.gov. Retrieved 2015-11-03.
- 1 2 Slain, Douglas (1999-03-01). "Lipid-Based Amphotericin B for the Treatment of Fungal Infections". Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 19 (3): 306–323. doi:10.1592/phco.19.4.306.30934. ISSN 1875-9114.
- ↑ Mistro, Sóstenes; Maciel, Isis de M.; Menezes, Rouseli G. de; Maia, Zuinara P.; Schooley, Robert T.; Badaró, Roberto (2012-06-15). "Does Lipid Emulsion Reduce Amphotericin B Nephrotoxicity? A Systematic Review and Meta-analysis". Clinical Infectious Diseases. 54 (12): 1774–1777. doi:10.1093/cid/cis290. ISSN 1058-4838. PMID 22491505.
- ↑ Pappas, Peter G.; Kauffman, Carol A.; Andes, David; Benjamin, Daniel K.; Calandra, Thierry F.; Edwards, John E.; Filler, Scott G.; Fisher, John F.; Kullberg, Bart-Jan (2009-03-01). "Clinical Practice Guidelines for the Management Candidiasis: 2009 Update by the Infectious Diseases Society of America". Clinical Infectious Diseases. 48 (5): 503–535. doi:10.1086/596757. ISSN 1058-4838. PMID 19191635.
- ↑ Groll, A. H.; Giri, N.; Petraitis, V.; Petraitiene, R.; Candelario, M.; Bacher, J. S.; Piscitelli, S. C.; Walsh, T. J. (2000-07-01). "Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system". The Journal of Infectious Diseases. 182 (1): 274–282. doi:10.1086/315643. ISSN 0022-1899. PMID 10882607.
- ↑ "Untitled Document". www.fungisome.com. Retrieved 2015-11-03.
- 1 2 "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Retrieved 2015-11-03.
- ↑ Wasan KM, Wasan EK, Gershkovich P, et al. (2009). "Highly Effective oral amphotericin B formulation against murine visceral leishmaniasis". J Infect Dis. 200 (3): 357–360. doi:10.1086/600105. PMID 19545212.
- ↑ Pappas, Peter G.; Kauffman, Carol A.; Andes, David; Benjamin, Daniel K.; Calandra, Thierry F.; Edwards, John E.; Filler, Scott G.; Fisher, John F.; Kullberg, Bart-Jan (2009-03-01). "Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America". Clinical Infectious Diseases. 48 (5): 503–535. doi:10.1086/596757. ISSN 1537-6591. PMID 19191635.
- ↑ Patel, Pratikkumar A.; Patravale, Vandana B. (2011). "AmbiOnp: solid lipid nanoparticles of amphotericin B for oral administration". Journal of Biomedical Nanotechnology. 7 (5): 632–639. doi:10.1166/jbn.2011.1332. PMID 22195480.
- ↑ Wasan, EK; Bartlett, K; Gershkovich, P; Sivak, O; Banno, B; Wong, Z; Gagnon, J; Gates, B; Leon, CG; Wasan, KM (2009). "Development and characterization of oral lipid-based amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans". International Journal of Pharmaceutics. 372 (1-2): 76–84. doi:10.1016/j.ijpharm.2009.01.003.
- ↑ Patel PA, Patravale,VB. AmbiOnp: solid lipid nanoparticles of amphotericin B for oral administration. Journal of Biomedical Nanotechnology.2011; 7(5):632-639
- ↑ Italia, JL; Yahya, MM; Singh, D (2009). "Biodegradable nanoparticles improve oral Bioavailability of Amphotericin B and Show Reduced Nephrotoxicity Compared to Intravenous Fungizone®". Pharmaceutical Research. 26 (6): 1324–1331. doi:10.1007/s11095-009-9841-2.
- ↑ AL-Quadeib, Bushra T.; Radwan, Mahasen A.; Siller, Lidija; Horrocks, Benjamin; Wright, Matthew C. (2015-07-01). "Stealth Amphotericin B nanoparticles for oral drug delivery: In vitro optimization". Saudi Pharmaceutical Journal. 23 (3): 290–302. doi:10.1016/j.jsps.2014.11.004. PMC 4475820
 . PMID 26106277.
. PMID 26106277. - ↑ Patel PA, Fernandes CB, Pol AS, Patravale VB. Oral Amphotericin B: Challenges and avenues. Int. J. Pharm. Biosci. Technol. 2013;1(1):1–9
- ↑ MedBullets.Org - Amphotericin B
- ↑ Walsh, Thomas J.; Finberg, Robert W.; Arndt, Carola; Hiemenz, John; Schwartz, Cindy; Bodensteiner, David; Pappas, Peter; Seibel, Nita; Greenberg, Richard N. (1999-03-11). "Liposomal Amphotericin B for Empirical Therapy in Patients with Persistent Fever and Neutropenia". New England Journal of Medicine. 340 (10): 764–771. doi:10.1056/NEJM199903113401004. ISSN 0028-4793. PMID 10072411.
- ↑ Perfect, John R.; Dismukes, William E.; Dromer, Francoise; Goldman, David L.; Graybill, John R.; Hamill, Richard J.; Harrison, Thomas S.; Larsen, Robert A.; Lortholary, Olivier (2010-02-01). "Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America". Clinical Infectious Diseases. 50 (3): 291–322. doi:10.1086/649858. ISSN 1058-4838. PMID 20047480.
- ↑ Jill Adler-Moore,* and Richard T. liposomal formulation, structure, mechanism of action and pre-clinical experience. Journal of Antimicrobial Chemotherapy (2002) 49, 21–30
- ↑ J. Czub, M. Baginski. Amphotericin B and Its New Derivatives Mode of action. Department of pharmaceutical Technology and Biochemistry. Faculty of Chemistry, Gdnsk University of Technology. 2009, 10-459-469.
- ↑ Zietse, R.; Zoutendijk, R.; Hoorn, E. J. "Fluid, electrolyte and acid–base disorders associated with antibiotic therapy". Nature Reviews Nephrology. 5 (4): 193–202. doi:10.1038/nrneph.2009.17.
- 1 2 Mesa-Arango, Ana Cecilia; Scorzoni, Liliana; Zaragoza, Oscar (2012-01-01). "It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug". Fungi and Their Interactions. 3: 286. doi:10.3389/fmicb.2012.00286. PMC 3441194
 . PMID 23024638.
. PMID 23024638. - ↑ O'Keeffe, Joseph; Doyle, Sean; Kavanagh, Kevin (2003-12-01). "Exposure of the yeast Candida albicans to the anti-neoplastic agent adriamycin increases the tolerance to amphotericin B". Journal of Pharmacy and Pharmacology. 55 (12): 1629–1633. doi:10.1211/0022357022359. ISSN 2042-7158.
- ↑ "Molecular modelling of amphotericin B-ergosterol primary complex in water II". Biophysical Chemistry. 141.
- 1 2 Baginski, M.; Czub, J. (2009). "Amphotericin B and Its New Derivatives–Mode of Action". Current Drug Metabolism. 10 (5): 459–69. doi:10.2174/138920009788898019. PMID 19689243.
- 1 2 Dutcher, James D. (1968-10-01). "THe discovery and development of amphotericin b". Chest. 54 (Supplement_1): 296–298. doi:10.1378/chest.54.Supplement_1.296. ISSN 0012-3692.
- 1 2 Laniado-Laborin R. and Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Revista Iberoamericana de Micologia. (2009): 223–7.
- ↑ Pfizer. Amphocin. Accessed at "Archived copy" (PDF). Archived from the original (PDF) on 2011-04-19. Retrieved 2010-02-18. on Feb 18 2010.
- ↑ "Differences in the Interaction of the Polyene Antibiotic Amphotericin B with Cholesterol- or Ergosterol-Containing Phospholipid Vesicles. A Circular Dichroism and Permeability Study". Biochemistry. 22.
- ↑ Drew, R. Pharmacology of amphotericin B. Uptodate. Sep 2009. Accessed at http://www.utdol.com/online/content/topic.do?topicKey=antibiot/4619&selectedTitle=2~150&source=search_result on Feb 18 2010.
- ↑ Khan N, Rawlings B, Caffrey P (2011-01-26). "A labile point in mutant amphotericin polyketide synthases". Biotechnol Lett. 33 (6): 1121–6. doi:10.1007/s10529-011-0538-3. PMID 21267757.
- ↑ McNamara, Carmel M.; Box, Stephen; Crawforth, James M.; Hickman, Benjamin S.; Norwood, Timothy J. (1998-01-01). "Biosynthesis of amphotericin B". Journal of the Chemical Society, Perkin Transactions 1: 83–88. doi:10.1039/A704545J.
- ↑ Caffrey, Patrick; Lynch, Susan; Flood, Elizabeth; Finnan, Shirley; Oliynyk, Markiyan (2001). "Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes". Chemistry & Biology. 8 (7): 713–723. doi:10.1016/S1074-5521(01)00046-1. PMID 11451671.
- ↑ McNamara, Carmel M.; Box, Stephen; Crawforth, James M.; Hickman, Benjamin S.; Norwood, Timothy J.; Rawlings, Bernard J. "Biosynthesis of amphotericin B". Journal of the Chemical Society, Perkin Transactions 1 (1): 83–88. doi:10.1039/a704545j.
- ↑ Nicolaou, K. C.; Daines, R. A.; Chakraborty, T. K.; Ogawa, Y. (1987-04-01). "Total synthesis of amphotericin B". Journal of the American Chemical Society. 109 (9): 2821–2822. doi:10.1021/ja00243a043. ISSN 0002-7863.
- ↑ "Halizon". Edu.drugs. Retrieved 2016-11-14.
External links
- AmBisome web site run by Astella Pharma
- "Special issue". Journal of Postgraduate Medicine. 51 (Suppl). 2005.
- Review Article: Oral Amphotericin B:Challenges and avenues
- AmBisome Summaries of Product Characteristics (United Kingdom)
