Polythiophene
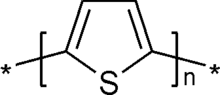

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. They can become conducting when oxidized. The study of polythiophenes and related conductive polymers was recognized by the awarding of the 2000 Nobel Prize in Chemistry to Alan J. Heeger, Alan MacDiarmid, and Hideki Shirakawa "for the discovery and development of conductive polymers". The most notable property of these materials, electrical conductivity, results from the delocalization of electrons along the polymer backbone – hence the term "synthetic metals". Conductivity however is not the only interesting property resulting from electron delocalization. The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes in solvent, temperature, applied potential, and binding to other molecules. Both color changes and conductivity changes are induced by the same mechanism—twisting of the polymer backbone, disrupting conjugation—making conjugated polymers attractive as sensors that can provide a range of optical and electronic responses.
A number of comprehensive reviews have been published on PTs, the earliest dating from 1981.[1] Schopf and Koßmehl published a comprehensive review of the literature published between 1990 and 1994.[2] Roncali surveyed electrochemical synthesis in 1992,[3] and the electronic properties of substituted PTs in 1997.[4] McCullough's 1998 review focussed on chemical synthesis of conducting PTs.[5] A general review of conjugated polymers from the 1990s was conducted by Reddinger and Reynolds in 1999.[6] Finally, Swager et al. examined conjugated-polymer-based chemical sensors in 2000.[7] These reviews are an excellent guide to the highlights of the primary PT literature from the last two decades.
Mechanism of conductivity and doping
Electrons are delocalized along the conjugated backbones of conducting polymers, usually through overlap of π-orbitals, resulting in an extended π-system with a filled valence band. By removing electrons from the π-system ("p-doping"), or adding electrons into the π-system ("n-doping"), a charged unit called a bipolaron is formed (see Figure 1).
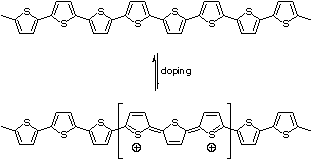
Doping is performed at much higher levels (20–40%) in conducting polymers than in semiconductors (<1%). The bipolaron moves as a unit along the polymer chain, and is responsible for the macroscopically observed conductivity of the polymer. For some samples of poly(3-dodecylthiophene) doped with iodine, the conductivity can approach 1000 S/cm.[8] (In comparison, the conductivity of copper is approximately 5×105 S/cm.) Generally, the conductivity of PTs is lower than 1000 S/cm, but high conductivity is not necessary for many applications of conducting polymers (see below for examples).
Simultaneous oxidation of the conducting polymer and introduction of counterions, p-doping, can be accomplished electrochemically or chemically. During the electrochemical synthesis of a PT, counterions dissolved in the solvent can associate with the polymer as it is deposited onto the electrode in its oxidized form. By doping the polymer as it is synthesized, a thick film can build up on an electrode—the polymer conducts electrons from the substrate to the surface of the film. Alternatively, a neutral conducting polymer film or solution can be doped post-synthesis.
Reduction of the conducting polymer, n-doping, is much less common than p-doping. An early study of electrochemical n-doping of poly(bithiophene) found that the n-doping levels are less than those of p-doping, the n-doping cycles were less efficient, the number of cycles required to reach maximum doping was higher, and the n-doping process appeared to be kinetically limited, possibly due to counterion diffusion in the polymer.[9]
A variety of reagents have been used to dope PTs. Iodine and bromine produce highly conductivitive materials,[8] which are unstable owing to slow evaporation of the halogenl.[10] Organic acids, including trifluoroacetic acid, propionic acid, and sulfonic acids produce PTs with lower conductivities than iodine, but with higher environmental stabilities.[10][11] Oxidative polymerization with ferric chloride can result in doping by residual catalyst,[12] although matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) studies have shown that poly(3-hexylthiophene)s are also partially halogenated by the residual oxidizing agent.[13] Poly(3-octylthiophene) dissolved in toluene can be doped by solutions of ferric chloride hexahydrate dissolved in acetonitrile, and can be cast into films with conductivities reaching 1 S/cm.[14] Other, less common p-dopants include gold trichloride[15] and trifluoromethanesulfonic acid.[16]
Structure and optical properties
Conjugation length and chromisms
The extended π-systems of conjugated PTs produce some of the most interesting properties of these materials—their optical properties. As an approximation, the conjugated backbone can be considered as a real-world example of the "electron-in-a-box" solution to the Schrödinger equation; however, the development of refined models to accurately predict absorption and fluorescence spectra of well-defined oligo(thiophene) systems is ongoing.[17] Conjugation relies upon overlap of the π-orbitals of the aromatic rings, which, in turn, requires the thiophene rings to be coplanar (see Figure 2, top).

The number of coplanar rings determines the conjugation length—the longer the conjugation length, the lower the separation between adjacent energy levels, and the longer the absorption wavelength. Deviation from coplanarity may be permanent, resulting from mislinkages during synthesis or especially bulky side chains; or temporary, resulting from changes in the environment or binding. This twist in the backbone reduces the conjugation length (see Figure 2, bottom), and the separation between energy levels is increased. This results in a shorter absorption wavelength.
Determining the maximum effective conjugation length requires the synthesis of regioregular PTs of defined length. The absorption band in the visible region is increasingly red-shifted as the conjugation length increases, and the maximum effective conjugation length is calculated as the saturation point of the red-shift. Early studies by ten Hoeve et al. estimated that the effective conjugation extended over 11 repeat units,[18] while later studies increased this estimate to 20 units.[19] The optical properties vary with chain length.[20] and 96-mer[21][21]
A variety of environmental factors can cause the conjugated backbone to twist, reducing the conjugation length and causing an absorption band shift, including solvent, temperature, application of an electric field, and dissolved ions. The absorption band of poly (3-thiophene acetic acid) in aqueous solutions of poly(vinyl alcohol) (PVA) shifts from 480 nm at pH 7 to 415 nm at pH 4. This is attributed to formation of a compact coil structure, which can form hydrogen bonds with PVA upon partial deprotonation of the acetic acid group.[22] Chiral PTs showed no induced circular dichroism (ICD) in chloroform, but displayed intense, but opposite, ICDs in chloroform–acetonitrile mixtures versus chloroform–acetone mixtures.[23] Also, a PT with a chiral amino acid side chain[24] displayed moderate absorption band shifts and ICDs, depending upon the pH and the concentration of buffer.[25]
Shifts in PT absorption bands due to changes in temperature result from a conformational transition from a coplanar, rodlike structure at lower temperatures to a nonplanar, coiled structure at elevated temperatures. For example, poly(3-(octyloxy)-4-methylthiophene) undergoes a color change from red–violet at 25 °C to pale yellow at 150 °C. An isosbestic point (a point where the absorbance curves at all temperatures overlap) indicates coexistence between two phases, which may exist on the same chain or on different chains.[26] Not all thermochromic PTs exhibit an isosbestic point: highly regioregular poly(3-alkylthiophene)s (PATs) show a continuous blue-shift with increasing temperature if the side chains are short enough so that they do not melt and interconvert between crystalline and disordered phases at low temperatures.
Finally, PTs can exhibit absorption shifts due to application of electric potentials (electrochromism),[27] or to introduction of alkali ions (ionochromism).[28] These effects will be discussed in the context of applications of PTs below.
Regioregularity
The asymmetry of 3-substituted thiophenes results in three possible couplings when two monomers are linked between the 2- and the 5-positions. These couplings are:
- 2,5', or head–tail (HT), coupling.
- 2,2', or head–head (HH), coupling
- 5,5', or tail–tail (TT), coupling
These three diads can be combined into four distinct triads, shown in Figure 3.

The triads are distinguishable by NMR spectroscopy, and the degree of regioregularity can be estimated by integration.[29][30]
Regioregularity affects the properties of PTs. A regiorandom copolymer of 3-methylthiophene and 3-butylthiophene possessed a conductivity of 50 S/cm, while a more regioregular copolymer with a 2:1 ratio of HT to HH couplings had a higher conductivity of 140 S/cm.[31] Films of regioregular poly(3-(4-octylphenyl)thiophene) (POPT) with greater than 94% HT content possessed conductivities of 4 S/cm, compared with 0.4 S/cm for regioirregular POPT.[32] PATs prepared using Rieke zinc formed "crystalline, flexible, and bronze-colored films with a metallic luster". On the other hand, the corresponding regiorandom polymers produced "amorphous and orange-colored films".[33] Comparison of the thermochromic properties of the Rieke PATs showed that, while the regioregular polymers showed strong thermochromic effects, the absorbance spectra of the regioirregular polymers did not change significantly at elevated temperatures. This was likely due to the formation of only weak and localized conformational defects. Finally, Xu and Holdcroft demonstrated that the fluorescence absorption and emission maxima of poly(3-hexylthiophene)s occur at increasingly lower wavelengths (higher energy) with increasing HH dyad content. The difference between absorption and emission maxima, the Stokes shift, also increases with HH dyad content, which they attributed to greater relief from conformational strain in the first excited state.[34]
Solubility
Unsubstituted PTs are conductive after doping, and have excellent environmental stability compared with some other conducting polymers such as polyacetylene, but are intractable and soluble only in solutions like mixtures of arsenic trifluoride and arsenic pentafluoride.[35] However, in 1987 examples of organic-soluble PTs were reported. Elsenbaumer et al., using a nickel-catalyzed Grignard cross-coupling, synthesized two soluble PTs, poly(3-butylthiophene) and poly(3-methylthiophene-'co'-3'-octylthiophene), which could be cast into films and doped with iodine to reach conductivities of 4 to 6 S/cm.[36] Hotta et al. synthesized poly(3-butylthiophene) and poly(3-hexylthiophene) electrochemically[37] (and later chemically[38]), and characterized the polymers in solution[39] and cast into films.[40] The soluble PATs demonstrated both thermochromism and solvatochromism (see above) in chloroform and 2,5-dimethyltetrahydrofuran.[41]
Water-soluble PT's are represented by sodium poly(3-thiophenealkanesulfonate)s.[42] In addition to conferring water solubility, the pendant sulfonate groups act as counterions, producing self-doped conducting polymers. Substituted PTs with tethered carboxylic acids,[43] acetic acids,[44] amino acids,[24] and urethanes[45] are also water-soluble.
Poly(3-(perfluorooctyl)thiophene)s soluble in supercritical carbon dioxide[46] were electrochemically and chemically synthesized by Collard et al.[47] Finally, unsubstituted oligothiophenes capped at both ends with thermally-labile alkyl esters were cast as films from solution, and then heated to remove the solublizing end groups. Atomic force microscopy (AFM) images showed a significant increase in long-range order after heating.[48]
Synthesis
PTs can be synthesized electrochemically, by applying a potential across a solution of the monomer, or chemically, using oxidants or cross-coupling catalysts. All methods have their advantages and disadvantages.
Electrochemical synthesis
In an electrochemical polymerization, a solution containing thiophene and an electrolyte produces a conductive PT film on the anode. Electrochemical polymerization is convenient, since the polymer does not need to be isolated and purified, but it can produce polymers with undesirable alpha-beta linkages and varying degrees of regioregularity.

As shown in Figure 4, oxidation of a monomer produces a radical cation, which can then couple with a second radical cation to form a dication dimer, or with another monomer to produce a radical cation dimer. Deposition of long, well-ordered chains onto the electrode surface is followed by growth of either long, flexible chains, or shorter, more crosslinked chains, depending upon the polymerization conditions.
The quality of an electrochemically prepared PT film is affected by a number of factors. These include the electrode material, current density, temperature, solvent, electrolyte, presence of water, and monomer concentration.[2] Two other important but interrelated factors are the structure of the monomer and the applied potential. The potential required to oxidize the monomer depends upon the electron density in the thiophene ring π-system. Electron-donating groups lower the oxidation potential, while electron-withdrawing groups increase the oxidation potential. Thus, 3-methylthiophene polymerizes in acetonitrile and tetrabutylammonium tetrafluoroborate at a potential of about 1.5 V vs. SCE (saturated calomel electrode), while unsubstituted thiophene polymerizes at about 1.7 V vs. SCE. Steric hindrance resulting from branching at the α-carbon of a 3-substituted thiophene inhibits polymerization.[49] This observation leads to the so-called "polythiophene paradox": the oxidation potential of many thiophene monomers is higher than the oxidation potential of the resulting polymer. In other words, the polymer can be irreversibly oxidized and decompose at a rate comparable to the polymerization of the corresponding monomer. This remains one of the major disadvantages of electrochemical polymerization, and limits its application for many thiophene monomers with complex side groups.
Chemical synthesis
Chemical synthesis offers two advantages compared with electrochemical synthesis of PTs: a greater selection of monomers, and, using the proper catalysts, the ability to synthesize perfectly regioregular substituted PTs. While PTs may have been chemically synthesized by accident more than a century ago,[50] the first planned chemical syntheses using metal-catalyzed polymerization of 2,5-dibromothiophene were reported by two groups independently in 1980. Yamamoto et al. used magnesium in tetrahydrofuran (THF) and nickel(bipyridine) dichloride, analogous to the Kumada coupling of Grignard reagents to aryl halides.[51] Lin and Dudek also used magnesium in THF, but with a series of acetylacetonate catalysts (Pd(acac)2, Ni(acac)2, Co(acac)2, and Fe(acac)3.[52]
Later developments produced higher molecular weight PTs than those initial efforts, and can be grouped into two categories based on their structure. Regioregular PTs can be synthesized by catalytic cross-coupling reactions of bromothiophenes, while polymers with varying degrees of regioregularity can be simply synthesized by oxidative polymerization.
Regioregular PTs were described by McCullough et al. in 1992.[53] As shown in Figure 5 (top),

selective bromination produces 2-bromo-3-alkylthiophene, which is followed by lithiation, transmetalation and then Kumada cross-coupling in the presence of a nickel catalyst. This method produces approximately 100% HT–HT couplings, according to NMR spectroscopy analysis of the diads. In the method subsequently described by Rieke et al. in 1993,[54] 2,5-dibromo-3-alkylthiophene is treated with highly reactive "Rieke zinc"[55] to form a mixture of organometallic isomers (Figure 5, bottom). Addition of a catalytic amount of Pd(PPh3)4 produces a regiorandom polymer, but treatment with Ni(dppe)Cl2 yields regioregular PAT in quantitative yield.[56]
While the McCullough and Rieke methods produce structurally homogenous PATs, they require low temperatures, the careful exclusion of water and oxygen, and brominated monomers. In contrast, the oxidative polymerization of thiophenes using ferric chloride described by Sugimoto in 1986 can be performed at room temperature under less demanding conditions.[57] This method has proven to be extremely popular; antistatic coatings are prepared on a commercial scale using ferric chloride (see below).[58]
A number of studies have been conducted in attempts to improve the yield and quality of the product obtained using the oxidative polymerization technique. In addition to ferric chloride, other oxidizing agents, including ferric chloride hydrate, copper perchlorate, and iron perchlorate have also been used successfully to polymerize 2,2'-bithiophene.[59] Slow addition of ferric chloride to the monomer solution produced poly(3-(4-octylphenyl)thiophene)s with approximately 94% H–T content.[32] Precipitation of ferric chloride in situ (in order to maximize the surface area of the catalyst) produced significantly higher yields and monomer conversions than adding monomer directly to crystalline catalyst.[60][61] Higher molecular weights were reported when dry air was bubbled through the reaction mixture during polymerization.[62] Exhaustive Soxhlet extraction after polymerization with polar solvents was found to effectively fractionate the polymer and remove residual catalyst before NMR spectroscopy.[29] Using a lower ratio of catalyst to monomer (2:1, rather than 4:1) may increase the regioregularity of poly(3-dodecylthiophene)s.[63] Andreani et al. reported higher yields of soluble poly(dialkylterthiophene)s in carbon tetrachloride rather than chloroform, which they attributed to the stability of the radical species in carbon tetrachloride.[64] Higher-quality catalyst, added at a slower rate and at reduced temperature, was shown to produce high molecular weight PATs with no insoluble polymer residue.[65] Laakso et al. used a factorial design to determine that increasing the ratio of catalyst to monomer increased the yield of poly(3-octylthiophene), and claimed that a longer polymerization time also increased the yield.[66]
The mechanism of the oxidative polymerization using ferric chloride has been controversial. Sugimoto et al. did not speculate on a mechanism in their 1986 report.[57] In 1992, Niemi et al. proposed a radical mechanism, shown in Figure 6(top).
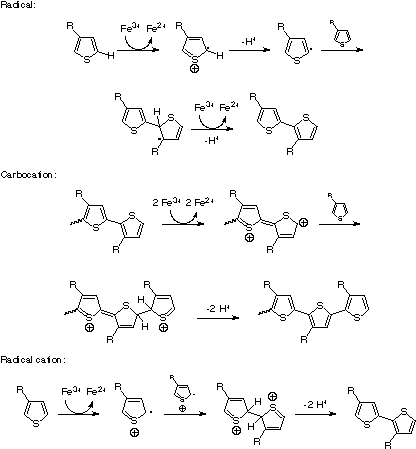
They based their mechanism on two assumptions. First, since they observed polymerization only in solvents where the catalyst was either partially or completely insoluble (chloroform, toluene, carbon tetrachloride, pentane, and hexane, and not diethyl ether, xylene, acetone, or formic acid), they concluded that the active sites of the polymerization must be at the surface of solid ferric chloride. Therefore, they discounted the possibilities of either two radical cations reacting with each other, or two radicals reacting with each other, "because the chloride ions at the surface of the crystal would prevent the radical cations or radicals from assuming positions suitable for dimerization".[67] Second, using 3-methylthiophene as a prototypical monomer, they performed quantum mechanical calculations to determine the energies and the total atomic charges on the carbon atoms of the four possible polymerization species (neutral 3-methylthiophene, the radical cation, the radical on carbon 2, and the radical on carbon 5).
Since the most negative carbon of the neutral 3-methylthiophene is also carbon 2, and the carbon with the highest odd electron population of the radical cation is carbon 2, they concluded that a radical cation mechanism would lead to mostly 2–2, H–H links. They then calculated the total energies of the species with the radicals at the 2 and the 5 carbons, and found that the latter was more stable by 1.5 kJ/mol. Therefore, the more stable radical could react with the neutral species, forming head-to-tail couplings as shown in Figure 6 (top).
Andersson et al. offered an alternative mechanism in the course of their studies of the polymerization of 3-(4-octylphenyl)thiophene with ferric chloride, where they found a high degree of regioregularity when the catalyst was added to the monomer mixture slowly. They concluded that, given the selectivity of the couplings, and the strong oxidizing conditions, the reaction could proceed via a carbocation mechanism (Figure 6, middle).[32]
The radical mechanism was directly challenged in a short communication in 1995, when Olinga and François noted that thiophene could be polymerized by ferric chloride in acetonitrile, a solvent in which the catalyst is completely soluble. Their analysis of the kinetics of thiophene polymerization also seemed to contradict the predictions of the radical polymerization mechanism.[68] Barbarella et al. studied the oligomerization of 3-(alkylsulfanyl)thiophenes, and concluded from their quantum mechanical calculations, and considerations of the enhanced stability of the radical cation when delocalized over a planar conjugated oligomer, that a radical cation mechanism analogous to that generally accepted for electrochemical polymerization was more likely (Figure 6, bottom).[69] Given the difficulties of studying a system with a heterogeneous, strongly oxidizing catalyst that produces difficult to characterize rigid-rod polymers, the mechanism of oxidative polymerization is by no means decided. However, the radical cation mechanism shown in Figure 6 is generally accepted as the most likely route for PT synthesis.
Applications

As an example of a static application, poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS) product Clevios P (Figure 7) from Heraeus has been extensively used as an antistatic coating (as packaging materials for electronic components, for example). AGFA coats 200 m × 10 m of photographic film per year with PEDOT:PSS because of its antistatic properties. The thin layer of PEDOT:PSS is virtually transparent and colorless, prevents electrostatic discharges during film rewinding, and reduces dust buildup on the negatives after processing.
PEDOT also have been proposed for dynamic applications where a potential is applied to a polymer film. PEDOT-coated windows and mirrors become opaque or reflective upon the application of an electric potential, a manifestation of its electrochromic properties.[27] Widespread adoption of electrochromic windows could save billions of dollars per year in air conditioning costs.[70] Phillips has commercialized a mobile phone with an electrically switchable PEDOT mirror.

Proposed applications
A number of applications have been proposed for conducting PTs, but have not achieved commercialization. Potential applications include field-effect transistors,[71] electroluminescent devices, solar cells, photochemical resists, nonlinear optic devices,[72] batteries, diodes, and chemical sensors.[73] In general, there are two categories of applications for conducting polymers. Static applications rely upon the intrinsic conductivity of the materials, combined with their ease of processing and material properties common to polymeric materials. Dynamic applications utilize changes in the conductive and optical properties, resulting either from application of electric potentials or from environmental stimuli.
PTs have been touted as sensor elements. In addition to biosensor applications, PTs can also be functionalized with receptors for detecting metal ions or chiral molecules as well. PTs with pendant crown ether functionalities exhibit properties that vary with the alkali metal.[74] and main-chain[28](Figure 8).

Chiral PTs in principle could be employed for detection of chiral analytes.[75][76][77][78] Yashima and Goto found that a PT with a chiral primary amine (Figure 9) [79][80]
Fluorinated polythiophene yield 7% efficiency in polymer-fullerene solar cells.[81]
References
- ↑ Street, G. B.; Clarke, T. C. (1981). "Conducting Polymers: A Review of Recent Work". IBM J. Res. Dev. 25: 51–57. doi:10.1147/rd.251.0051.
- 1 2 Schopf, G.; Koßmehl, G. (1997). "Polythiophenes—Electrically Conducting Polymers". Adv. Polym. Sci. Advances in Polymer Science. 129: 1–166. doi:10.1007/BFb0008700. ISBN 3-540-61857-0.
- ↑ Roncali, Jean (1992). "Conjugated poly(thiophenes): synthesis, functionalization, and applications". Chemical Reviews. 92 (4): 711. doi:10.1021/cr00012a009.
- ↑ Roncali, Jean (1997). "Synthetic Principles for Bandgap Control in Linear π-Conjugated Systems". Chemical Reviews. 97 (1): 173–206. doi:10.1021/cr950257t. PMID 11848868.
- ↑ McCullough, Richard D. (1998). "The Chemistry of Conducting Polythiophenes". Advanced Materials. 10 (2): 93. doi:10.1002/(SICI)1521-4095(199801)10:2<93::AID-ADMA93>3.0.CO;2-F.
- ↑ Reddinger, J. L.; Reynolds, J. R. (1999). "Molecular Engineering of p-Conjugated Polymers". Adv. Polym. Sci. Advances in Polymer Science. 145: 57–122. doi:10.1007/3-540-70733-6_2. ISBN 978-3-540-65210-6.
- ↑ McQuade, D. Tyler; Pullen, Anthony E.; Swager, Timothy M. (2000). "Conjugated Polymer-Based Chemical Sensors". Chemical Reviews. 100 (7): 2537–74. doi:10.1021/cr9801014. PMID 11749295.
- 1 2 McCullough, Richard D.; Tristram-Nagle, Stephanie; Williams, Shawn P.; Lowe, Renae D.; Jayaraman, Manikandan (1993). "Self-orienting head-to-tail poly(3-alkylthiophenes): new insights on structure-property relationships in conducting polymers". Journal of the American Chemical Society. 115 (11): 4910. doi:10.1021/ja00064a070.
- ↑ Mastragostino, M.; Soddu, L. (1990). "Electrochemical characterization of "n" doped polyheterocyclic conducting polymers—I. Polybithiophene". Electrochimica Acta. 35 (2): 463. doi:10.1016/0013-4686(90)87029-2.
- 1 2 Loponen, M.; Taka, T.; Laakso, J.; Vakiparta, K.; Suuronen, K.; Valkeinen, P.; Osterholm, J. (1991). "Doping and dedoping processes in poly (3-alkylthiophenes)". Synthetic Metals. 41: 479. doi:10.1016/0379-6779(91)91111-M.
- ↑ Bartuš, Ján (1991). "Electrically Conducting Thiophene Polymers". Journal of Macromolecular Science: Part A – Chemistry. 28 (9): 917–924. doi:10.1080/00222339108054069.
- ↑ Qiao, X.; Wang, Xianhong; Mo, Zhishen (2001). "The FeCl3-doped poly(3-alkyithiophenes) in solid state". Synthetic Metals. 122 (2): 449. doi:10.1016/S0379-6779(00)00587-7.
- ↑ McCarley, Tracy Donovan; Noble; Dubois, C. J.; McCarley, Robin L. (2001). "MALDI-MS Evaluation of Poly(3-hexylthiophene) Synthesized by Chemical Oxidation with FeCl3". Macromolecules. 34 (23): 7999. doi:10.1021/ma002140z.
- ↑ Heffner, G.; Pearson, D. (1991). "Solution processing of a doped conducting polymer". Synthetic Metals. 44 (3): 341. doi:10.1016/0379-6779(91)91821-Q.
- ↑ S.a. Abdou, M.; Holdcroft, Steven (1993). "Oxidation of π-conjugated polymers with gold trichloride: enhanced stability of the electronically conducting state and electroless deposition of Au0". Synthetic Metals. 60 (2): 93. doi:10.1016/0379-6779(93)91226-R.
- ↑ Rudge, Andy; Raistrick, Ian; Gottesfeld, Shimshon; Ferraris, John P. (1994). "A study of the electrochemical properties of conducting polymers for application in electrochemical capacitors". Electrochimica Acta. 39 (2): 273. doi:10.1016/0013-4686(94)80063-4.
- ↑ Bässler, H. "Electronic Excitation". In Electronic Materials: The Oligomer Approach; Müllen, K.; Wegner, G., Eds.; Wiley-VCH: Weinheim, Germany, 1998, ISBN 3-527-29438-4
- ↑ Ten Hoeve, W.; Wynberg, H.; Havinga, E. E.; Meijer, E. W. (1991). "Substituted .. – undecithiophenes, the longest characterized oligothiophenes". Journal of the American Chemical Society. 113 (15): 5887. doi:10.1021/ja00015a067.
- ↑ Meier, H.; Stalmach, U.; Kolshorn, H (September 1997). "Effective conjugation length and UV/vis spectra of oligomers". Acta Polymerica. 48 (9): 379–384. doi:10.1002/actp.1997.010480905.
- ↑ Nakanishi, Hidetaka; Sumi, Naoto; Aso, Yoshio; Otsubo, Tetsuo (1998). "Synthesis and Properties of the Longest Oligothiophenes: the Icosamer and Heptacosamer". The Journal of Organic Chemistry. 63 (24): 8632. doi:10.1021/jo981541y.
- 1 2 Izumi, Tsuyoshi; Kobashi, Seiji; Takimiya, Kazuo; Aso, Yoshio; Otsubo, Tetsuo (2003). "Synthesis and Spectroscopic Properties of a Series of β-Blocked Long Oligothiophenes up to the 96-mer: Revaluation of Effective Conjugation Length". Journal of the American Chemical Society. 125 (18): 5286–7. doi:10.1021/ja034333i. PMID 12720435.
- ↑ De Souza, J.; Pereira, Ernesto C. (2001). "Luminescence of poly(3-thiopheneacetic acid) in alcohols and aqueous solutions of poly(vinyl alcohol)". Synthetic Metals. 118: 167. doi:10.1016/S0379-6779(00)00453-7.
- ↑ Goto, H.; Yashima, E.; Okamoto, Y. (2000). "Unusual solvent effects on chiroptical properties of an optically active regioregular polythiophene in solution". Chirality. 12 (5–6): 396–399. doi:10.1002/(SICI)1520-636X(2000)12:5/6<396::AID-CHIR17>3.0.CO;2-X. PMID 10824159.
- 1 2 Andersson, M.; Ekeblad, P. O.; Hjertberg, T.; Wennerström, O.; Inganäs, O. (1991). "Polythiophene with a free amino-acid side-chain". Polym. Commun. 32: 546–548.
- ↑ Nilsson, K. P. R.; Andersson, M. R.; Inganäs, O. (2002). "Conformational transitions of a free amino-acid-functionalized polythiophene induced by different buffer systems". Journal of Physics: Condensed Matter. 14 (42): 10011. doi:10.1088/0953-8984/14/42/313.
- ↑ Roux, Claudine; Leclerc, Mario (1992). "Rod-to-coil transition in alkoxy-substituted polythiophenes". Macromolecules. 25 (8): 2141. doi:10.1021/ma00034a012.
- 1 2 H. W. Heuer; R. Wehrmann; S. Kirchmeyer (2002). "Electrochromic Window Based on Conducting Poly(3,4-ethylenedioxythiophene)-Poly(styrene sulfonate)". Advanced Functional Materials. 12 (2): 89. doi:10.1002/1616-3028(20020201)12:2<89::AID-ADFM89>3.0.CO;2-1.
- 1 2 Marsella, Michael J.; Swager, Timothy M. (1993). "Designing conducting polymer-based sensors: selective ionochromic response in crown ether-containing polythiophenes". Journal of the American Chemical Society. 115 (25): 12214. doi:10.1021/ja00078a090.
- 1 2 Barbarella, Giovanna; Bongini, Alessandro; Zambianchi, Massimo (1994). "Regiochemistry and Conformation of Poly(3-hexylthiophene) via the Synthesis and the Spectroscopic Characterization of the Model Configurational Triads". Macromolecules. 27 (11): 3039. doi:10.1021/ma00089a022.
- ↑ Diaz-Quijada, G. A.; et al. (1996). "Regiochemical Analysis of Water Soluble Conductive Polymers: Sodium Poly(ω-(3-thienyl)alkanesulfonates)". Macromolecules. 29 (16): 5416. doi:10.1021/ma960126.
- ↑ Elsenbaumer, R. L.; Jen, K.-Y.; Miller, G. G.; Eckhardt, H.; Shacklette, L. W.; Jow, R. "Poly (alkyl thiophenes) and Poly (substituted heteroaromatic vinylenes): Versatile, Highly Conductive, Processible Polymers with Tunable Properties". In Electronic Properties of Conjugated Polymers (Eds: Kuzmany, H.; Mehring, M.; Roth, S.), Springer, Berlin, 1987, ISBN 0-387-18582-8
- 1 2 3 Andersson, M. R.; Selse, D.; Berggren, M.; Jaervinen, H.; Hjertberg, T.; Inganaes, O.; Wennerstroem, O.; Oesterholm, J.-E. (1994). "Regioselective polymerization of 3-(4-octylphenyl)thiophene with FeCl3". Macromolecules. 27 (22): 6503. doi:10.1021/ma00100a039.
- ↑ Chen, Tian-An; Wu, Xiaoming; Rieke, Reuben D. (1995). "Regiocontrolled Synthesis of Poly(3-alkylthiophenes) Mediated by Rieke Zinc: Their Characterization and Solid-State Properties". Journal of the American Chemical Society. 117: 233. doi:10.1021/ja00106a027.
- ↑ Xu, Bai; Holdcroft, Steven (1993). "Molecular control of luminescence from poly(3-hexylthiophenes)". Macromolecules. 26 (17): 4457. doi:10.1021/ma00069a009.
- ↑ Frommer, Jane E. (1986). "Conducting polymer solutions". Accounts of Chemical Research. 19: 2. doi:10.1021/ar00121a001.
- ↑ Elsenbaumer, R.; Jen, K.; Oboodi, R. (1986). "Processible and environmentally stable conducting polymers☆". Synthetic Metals. 15 (2–3): 169. doi:10.1016/0379-6779(86)90020-2.
- ↑ Hotta, S.; Rughooputh, S. D. D. V.; Heeger, A. J.; Wudl, F. (1987). "Spectroscopic studies of soluble poly(3-alkylthienylenes)". Macromolecules. 20: 212. doi:10.1021/ma00167a038.
- ↑ Hotta, S.; Soga, M.; Sonoda, N. (1988). "Novel organosynthetic routes to polythiophene and its derivatives". Synthetic Metals. 26 (3): 267. doi:10.1016/0379-6779(88)90243-3.
- ↑ Hotta, S. (1987). "Electrochemical synthesis and spectroscopic study of poly(3-alkylthienylenes)". Synthetic Metals. 22 (2): 103. doi:10.1016/0379-6779(87)90528-5.
- ↑ Hotta, S.; Rughooputh, S.; Heeger, A. (1987). "Conducting polymer composites of soluble polythiophenes in polystyrene". Synthetic Metals. 22: 79. doi:10.1016/0379-6779(87)90573-X.
- ↑ Rughooputh, S. D. D. V.; Hotta, S.; Heeger, A. J.; Wudl, F. (May 1987). "Chromism of soluble polythienylenes". Journal of Polymer Science Part B: Polymer Physics. 25 (5): 1071–1078. doi:10.1002/polb.1987.090250508.
- ↑ Patil, A. O.; Ikenoue, Y.; Wudl, Fred; Heeger, A. J. (1987). "Water soluble conducting polymers". Journal of the American Chemical Society. 109 (6): 1858. doi:10.1021/ja00240a044.
- ↑ Englebienne, Patrick; Weiland, Mich le (1996). "Synthesis of water-soluble carboxylic and acetic acid-substituted poly(thiophenes) and the application of their photochemical properties in homogeneous competitive immunoassays". Chemical Communications (14): 1651. doi:10.1039/cc9960001651.
- ↑ Kim; Chen, Li; Gong; Osada, Yoshihito (1999). "Titration Behavior and Spectral Transitions of Water-Soluble Polythiophene Carboxylic Acids". Macromolecules. 32 (12): 3964. doi:10.1021/ma981848z.
- ↑ Jung, S.; Hwang, D.-H.; Zyung, T.; Kim, W. H.; Chittibabu, K. G.; Tripathy, S. K. (1998). "Temperature dependent photoluminescence and electroluminescence properties of polythiophene with hydrogen bonding side chain". Synthetic Metals. 98 (2): 107. doi:10.1016/S0379-6779(98)00161-1.
- ↑ Desimone, J. M.; Guan, Z.; Elsbernd, C. S. (1992). "Synthesis of Fluoropolymers in Supercritical Carbon Dioxide". Science. 257 (5072): 945–7. doi:10.1126/science.257.5072.945. PMID 17789638.
- ↑ Li, L.; Counts, K. E.; Kurosawa, S.; Teja, A. S.; Collard, D. M. (2004). "Tuning the Electronic Structure and Solubility of Conjugated Polymers with Perfluoroalkyl Substituents: Poly(3-perfluorooctylthiophene), the First Supercritical CO2-soluble Conjugated Polymer". Advanced Materials. 16 (2): 180. doi:10.1002/adma.200305333.
- ↑ Murphy, Amanda R.; Fréchet, Jean M. J.; Chang, Paul; Lee, Josephine; Subramanian, Vivek (2004). "Organic Thin Film Transistors from a Soluble Oligothiophene Derivative Containing Thermally Removable Solubilizing Groups". Journal of the American Chemical Society. 126 (6): 1596–7. doi:10.1021/ja039529x. PMID 14871066.
- ↑ Roncali, J.; Garreau, R.; Yassar, A.; Marque, P.; Garnier, F.; Lemaire, M. (1987). "Effects of steric factors on the electrosynthesis and properties of conducting poly(3-alkylthiophenes)". The Journal of Physical Chemistry. 91 (27): 6706. doi:10.1021/j100311a030.
- ↑ Meyer, Victor (January–June 1883). "Ueber den Begleiter des Benzols im Steinkohlentheer" [On the companion of benzene in stone coal]. Berichte der deutschen chemischen Gesellschaft (in German). 16 (1): 1465–1478. doi:10.1002/cber.188301601324.
- ↑ Yamamoto, Takakazu; Sanechika, Kenichi; Yamamoto, Akio (January 1980). "Preparation of thermostable and electric-conducting poly(2,5-thienylene)". Journal of Polymer Science: Polymer Letters Edition. 18 (1): 9–12. doi:10.1002/pol.1980.130180103.
- ↑ Lin, John W-P.; Dudek, Lesley P. (September 1980). "Synthesis and properties of poly(2,5-thienylene)". Journal of Polymer Science: Polymer Chemistry Edition. 18 (9): 2869–2873. doi:10.1002/pol.1980.170180910.
- ↑ McCullough, Richard D.; Lowe, Renae D. (1992). "Enhanced electrical conductivity in regioselectively synthesized poly(3-alkylthiophenes)". Journal of the Chemical Society, Chemical Communications: 70. doi:10.1039/C39920000070.
- ↑ Chen, Tian An; O'Brien, Richard A.; Rieke, Reuben D. (1993). "Use of highly reactive zinc leads to a new, facile synthesis for polyarylenes". Macromolecules. 26 (13): 3462. doi:10.1021/ma00065a036.
- ↑ Zhu, Lishan; Wehmeyer, Richard M.; Rieke, Reuben D. (1991). "The direct formation of functionalized alkyl(aryl)zinc halides by oxidative addition of highly reactive zinc with organic halides and their reactions with acid chlorides, .alpha.,.beta.-unsaturated ketones, and allylic, aryl, and vinyl halides". The Journal of Organic Chemistry. 56 (4): 1445. doi:10.1021/jo00004a021.
- ↑ Chen, Tian An; Rieke, Reuben D. (1992). "The first regioregular head-to-tail poly(3-hexylthiophene-2,5-diyl) and a regiorandom isopolymer: nickel versus palladium catalysis of 2(5)-bromo-5(2)-(bromozincio)-3-hexylthiophene polymerization". Journal of the American Chemical Society. 114 (25): 10087. doi:10.1021/ja00051a066.
- 1 2 Sugimoto, R.; Taketa, S.; Gu, H. B.; Yoshino, K (1986). "Preparation of soluble polythiophene derivatives utilizing transition metal halides as catalysts and their property". Chemistry Express. 1 (11): 635–638.
- ↑ Jonas, F.; Heywang, G.; Schmidtberg, W.; Heinze, J.; Dietrich, M. U.S. Patent 5,035,926, 1991.
- ↑ Ruckenstein, E.; Park, J. (1991). "Polythiophene and polythiophene-based conducting composites". Synthetic Metals. 44 (3): 293. doi:10.1016/0379-6779(91)91817-T.
- ↑ Costa Bizzarri, P.; Andreani, Franco; Della Casa, Carlo; Lanzi, Massimiliano; Salatelli, Elisabetta (1995). "Ester-functionalized poly(3-alkylthienylene)s: substituent effects on the polymerization with FeCl3". Synthetic Metals. 75 (2): 141. doi:10.1016/0379-6779(95)03401-5.
- ↑ Fraleoni-Morgera, Alessandro; Della-Casa, Carlo; Lanzi, Massimiliano; Costa-Bizzarri, Paolo (2003). "Investigation on Different Procedures in the Oxidative Copolymerization of a Dye-Functionalized Thiophene with 3-Hexylthiophene". Macromolecules. 36 (23): 8617. doi:10.1021/ma0348730.
- ↑ Pomerantz, M.; Tseng, J.; Zhu, H.; Sproull, S.; Reynolds, J.; Uitz, R.; Arnott, H.; Haider, M. (1991). "Processable polymers and copolymers of 3-alkylthiophenes and their blends". Synthetic Metals. 41 (3): 825. doi:10.1016/0379-6779(91)91505-5.
- ↑ Qiao, X.; Wang, Xianhong; Zhao, Xiaojiang; Liu, Jian; Mo, Zhishen (2000). "Poly(3-dodecylthiophenes) polymerized with different amounts of catalyst". Synthetic Metals. 114 (3): 261. doi:10.1016/S0379-6779(00)00233-2.
- ↑ Andreani, F.; Salatelli, E.; Lanzi, M. (February 1996). "Novel poly(3,3 – and 3',4'-dialkyl- 2,2':5',2 – terthiophene)s by chemical oxidative synthesis: evidence for a new step towards the optimization of this process". Polymer. 37 (4): 661–665. doi:10.1016/0032-3861(96)83153-3.
- ↑ Gallazzi, M.; Bertarelli, C.; Montoneri, E. (2002). "Critical parameters for product quality and yield in the polymerisation of 3,3"-didodecyl-2,2′:5′,2"-terthiophene". Synthetic Metals. 128: 91. doi:10.1016/S0379-6779(01)00665-8.
- ↑ Laakso, J.; Jarvinen, H.; Skagerberg, B. (1993). "Recent developments in the polymerization of 3-alkylthiophenes". Synthetic Metals. 55 (2–3): 1204. doi:10.1016/0379-6779(93)90225-L.
- ↑ Niemi, V. M.; Knuuttila, P.; Österholm, J. E.; Korvola, J. (1992). "Polymerization of 3-alkylthiophenes with ferric chloride". Polymer. 33 (7): 1559–1562. doi:10.1016/0032-3861(92)90138-M..
- ↑ Olinga, T.; François, B. (1995). "Kinetics of polymerization of thiophene by FeCl3 in choloroform and acetonitrile". Synthetic Metals. 69: 297. doi:10.1016/0379-6779(94)02457-A.
- ↑ Barbarella, Giovanna; Zambianchi, Massimo; Di Toro, Rosanna; Colonna, Martino; Iarossi, Dario; Goldoni, Francesca; Bongini, Alessandro (1996). "Regioselective Oligomerization of 3-(Alkylsulfanyl)thiophenes with Ferric Chloride". The Journal of Organic Chemistry. 61 (23): 8285–8292. doi:10.1021/jo960982j. PMID 11667817.
- ↑ Rosseinsky, D. R.; Mortimer, R. J. (2001). "Electrochromic Systems and the Prospects for Devices". Advanced Materials. 13 (11): 783. doi:10.1002/1521-4095(200106)13:11<783::AID-ADMA783>3.0.CO;2-D.
- ↑ Garnier, F. "Field-Effect Transistors Based on Conjugated Materials". In Electronic Materials: The Oligomer Approach (Eds: Müllen, K.; Wegner, G.), Wiley-VCH, Weinheim, 1998, ISBN 3-527-29438-4
- ↑ Harrison, M. G.; Friend, R. H. "Optical Applications". In Electronic Materials: The Oligomer Approach (Eds: Müllen, K.; Wegner, G.), Wiley-VCH, Weinheim, 1998, ISBN 3-527-29438-4
- ↑ Martina, V; Ionescu, K.; Pigani, L; Terzi, F; Ulrici, A.; Zanardi, C.; Seeber, R (March 2007). "Development of an electronic tongue based on a PEDOT-modified voltammetric sensor". Analytical and Bioanalytical Chemistry. 387 (6): 2101–2110. doi:10.1007/s00216-006-1102-1. PMID 17235499.
- ↑ Bäuerle, Peter; Scheib, Stefan (1993). "Molecular recognition of alkali-ions by crown-ether-functionalized poly(alkylthiophenes)". Advanced Materials. 5 (11): 848. doi:10.1002/adma.19930051113.
- ↑ Goto, Hidetoshi; Okamoto, Yoshio; Yashima, Eiji (2002). "Metal-Induced Supramolecular Chirality in an Optically Active Polythiophene Aggregate". Chemistry: A European Journal. 8 (17): 4027–36. doi:10.1002/1521-3765(20020902)8:17<4027::AID-CHEM4027>3.0.CO;2-Q. PMID 12360944.
- ↑ Goto, Hidetoshi; Okamoto, Yoshio; Yashima, Eiji (2002). "Solvent-Induced Chiroptical Changes in Supramolecular Assemblies of an Optically Active, Regioregular Polythiophene". Macromolecules. 35 (12): 4590. doi:10.1021/ma012083p.
- ↑ Goto, Hidetoshi; Yashima, Eiji (2002). "Electron-Induced Switching of the Supramolecular Chirality of Optically Active Polythiophene Aggregates". Journal of the American Chemical Society. 124 (27): 7943–9. doi:10.1021/ja025900p. PMID 12095338.
- ↑ Sakurai, Shin-Ichiro; Goto, Hidetoshi; Yashima, Eiji (2001). "Synthesis and Chiroptical Properties of Optically Active, Regioregular Oligothiophenes". Organic Letters. 3 (15): 2379–82. doi:10.1021/ol016189g. PMID 11463321.
- ↑ Yashima, Eiji; Goto, Hidetoshi; Okamoto, Yoshio (1999). "Metal-Induced Chirality Induction and Chiral Recognition of Optically Active, Regioregular Polythiophenes". Macromolecules. 32 (23): 7942. doi:10.1021/ma9912305.
- ↑ Lemaire, Marc; Delabouglise, Didier; Garreau, Robert; Guy, Alain; Roncali, Jean (1988). "Enantioselective chiral poly(thiophenes)". Journal of the Chemical Society, Chemical Communications (10): 658. doi:10.1039/C39880000658.
- ↑ Price, Samuel C.; Stuart, Andrew C.; Yang, Liqiang; Zhou, Huaxing; You, Wei (2011). "Fluorine Substituted Conjugated Polymer of Medium Band Gap Yields 7% Efficiency in Polymer−Fullerene Solar Cells". Journal of the American Chemical Society. 133 (12): 4625–4631. doi:10.1021/ja1112595. PMID 21375339.
Further reading
- Handbook of Conducting Polymers (Eds: T. A. Skotheim, R. L. Elsenbaumer, J. R. Reynolds), Marcel Dekker, New York, 1998. ISBN 0-8247-0050-3.
- G. Schopf, G. Koßmehl, Polythiophenes: Electrically Conductive Polymers, Springer, Berlin, 1997, ISBN 3-540-61483-4; ISBN 0-387-61483-4.
- Synthetic Metals (journal). ISSN 0379-6779.