Carbon tetrachloride
| |||
| | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Carbon tetrachloride, Tetrachloromethane | |||
| Other names
Benziform, Benzinoform, Carbon chloride, Carbon tet, Freon-10, Refrigerant-10, Halon-104, Methane tetrachloride, Methyl tetrachloride, Perchloromethane, Tetraform, Tetrasol | |||
| Identifiers | |||
| 56-23-5 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:27385 | ||
| ChEMBL | ChEMBL44814 | ||
| ChemSpider | 5730 | ||
| ECHA InfoCard | 100.000.239 | ||
| EC Number | 200-262-8 | ||
| KEGG | C07561 | ||
| PubChem | 5943 | ||
| RTECS number | FG4900000 | ||
| UNII | CL2T97X0V0 | ||
| UN number | 1846 | ||
| |||
| |||
| Properties | |||
| CCl4 | |||
| Molar mass | 153.81 g·mol−1 | ||
| Appearance | colourless liquid | ||
| Odor | ether-like odor | ||
| Density | 1.5867 g cm−3 (liquid) 1.831 g cm−3 at −186 °C (solid) | ||
| Melting point | −22.92 °C (−9.26 °F; 250.23 K) | ||
| Boiling point | 76.72 °C (170.10 °F; 349.87 K) | ||
| 0.097 g/100 mL (0 °C) 0.081 g/100 mL (25 °C) | |||
| Solubility | soluble in alcohol, ether, chloroform, benzene, naphtha, CS2, formic acid | ||
| log P | 2.64 | ||
| Vapor pressure | 11.94 kPa at 20 °C | ||
| Henry's law constant (kH) |
2.76x10−2 atm-cu m/mol | ||
| Refractive index (nD) |
1.4607 | ||
| 0 D | |||
| Structure | |||
| Monoclinic | |||
| Tetragonal | |||
| Tetrahedral | |||
| 0 D | |||
| Thermochemistry | |||
| 132.6 J/mol K | |||
| Std molar entropy (S |
214.42 J/mol K | ||
| Std enthalpy of formation (ΔfH |
-139.3 kJ/mol | ||
| Gibbs free energy (ΔfG˚) |
-686 kJ/mol | ||
| Hazards | |||
| Safety data sheet | See: data page ICSC 0024 | ||
| EU classification (DSD) |
| ||
| R-phrases | R23/24/25, R40, R48/23, R59, R52/53 | ||
| S-phrases | (S1/2), S23, S36/37, S45, S59, S61 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| 982 °C (1,800 °F; 1,255 K) | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
2350 mg/kg | ||
| LC50 (median concentration) |
5400 ppm (mammal) 8000 ppm (rat, 4 hr) 9526 ppm (mouse, 8 hr)[1] | ||
| LCLo (lowest published) |
1000 ppm (human) 20,000 ppm (guinea pig, 2 hr) 38,110 ppm (cat, 2 hr) 50,000 ppm (human, 5 min) 14,620 ppm (dog, 8 hr)[1] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 10 ppm C 25 ppm 200 ppm (5-minute maximum peak in any 4 hours)[2] | ||
| REL (Recommended) |
Ca ST 2 ppm (12.6 mg/m3) [60-minute][2] | ||
| IDLH (Immediate danger) |
200 ppm[2] | ||
| Related compounds | |||
| Other cations |
Silicon tetrachloride Germanium tetrachloride Tin tetrachloride Lead tetrachloride | ||
| Related chloromethanes |
Chloromethane Dichloromethane Chloroform | ||
| Related compounds |
Tetrafluoromethane Tetrabromomethane Tetraiodomethane | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
| Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Carbon tetrachloride, also known by many other names (the most notable being tetrachloromethane, also recognized by the IUPAC), carbon tet in the cleaning industry, Halon-104 in firefighting and Refrigerant-10 in HVACR, is an organic compound with the chemical formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It has practically no flammability at lower temperatures.
History and synthesis
Carbon tetrachloride was originally synthesized by the French chemist Henri Victor Regnault in 1839 by the reaction of chloroform with chlorine,[3] but now it is mainly produced from methane:
- CH4 + 4 Cl2 → CCl4 + 4 HCl
The production often utilizes by-products of other chlorination reactions, such as from the syntheses of dichloromethane and chloroform. Higher chlorocarbons are also subjected to "chlorinolysis":
- C2Cl6 + Cl2 → 2 CCl4
Prior to the 1950s, carbon tetrachloride was manufactured by the chlorination of carbon disulfide at 105 to 130 °C:[4]
The production of carbon tetrachloride has steeply declined since the 1980s due to environmental concerns and the decreased demand for CFCs, which were derived from carbon tetrachloride. In 1992, production in the U.S./Europe/Japan was estimated at 720,000 tonnes.[4]
Properties
In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetrical geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane. As a solvent, it is well suited to dissolving other non-polar compounds, fats, and oils. It can also dissolve iodine. It is somewhat volatile, giving off vapors with a smell characteristic of other chlorinated solvents, somewhat similar to the tetrachloroethylene smell reminiscent of dry cleaners' shops.
Solid tetrachloromethane has two polymorphs: crystalline II below −47.5 °C (225.6 K) and crystalline I above −47.5 °C.[5]
At −47.3 °C it has monoclinic crystal structure with space group C2/c and lattice constants a = 20.3, b = 11.6, c = 19.9 (.10−1 nm), β = 111°.[6] With a specific gravity greater than 1, carbon tetrachloride will be present as a dense nonaqueous phase liquid if sufficient quantities are spilled in the environment.
Uses
In organic chemistry, carbon tetrachloride serves as a source of chlorine in the Appel reaction.
Historic uses
Prior to the Montreal Protocol, large quantities of carbon tetrachloride were used to produce the chlorofluorocarbon refrigerants R-11 (trichlorofluoromethane) and R-12 (dichlorodifluoromethane). However, these refrigerants play a role in ozone depletion and have been phased out. Carbon tetrachloride is still used to manufacture less destructive refrigerants. Carbon tetrachloride has also been used in the detection of neutrinos.
Solvent
It once was a popular solvent in organic chemistry, but, because of its adverse health effects, it is rarely used today.[7] It is sometimes useful as a solvent for infrared spectroscopy, because there are no significant absorption bands > 1600 cm−1. Because carbon tetrachloride does not have any hydrogen atoms, it was historically used in proton NMR spectroscopy. In addition to being toxic, its dissolving power is low.[8] Its use has been largely superseded by deuterated solvents. Use of carbon tetrachloride in determination of oil has been replaced by various other solvents, such as tetrachloroethylene.[7] Because it has no C-H bonds, carbon tetrachloride does not easily undergo free-radical reactions. It is a useful solvent for halogenations either by the elemental halogen or by a halogenation reagent such as N-bromosuccinimide (these conditions are known as Wohl-Ziegler Bromination).
Fire suppression

In 1910, the Pyrene Manufacturing Company of Delaware filed a patent to use carbon tetrachloride to extinguish fires.[9] The liquid was vaporized by the heat of combustion and extinguished flames, an early form of gaseous fire suppression. At the time it was believed the gas simply displaced oxygen in the area near the fire, but later research found that the gas actually inhibits the chemical chain reaction of the combustion process.
In 1911, Pyrene patented a small, portable extinguisher that used the chemical.[10] The extinguisher consisted of a brass bottle with an integrated handpump that was used to expel a jet of liquid toward the fire. As the container was unpressurized, it could easily be refilled after use.[11] Carbon tetrachloride was suitable for liquid and electrical fires and the extinguishers were often carried on aircraft or motor vehicles.
In the first half of the 20th century, another common fire extinguisher was a single-use, sealed glass globe known as a "fire grenade," filled with either carbon tetrachloride or salt water. The bulb could be thrown at the base of the flames to quench the fire. The carbon tetrachloride type could also be installed in a spring-loaded wall fixture with a solder-based restraint. When the solder melted by high heat, the spring would either break the globe or launch it out of the bracket, allowing the extinguishing agent to be automatically dispersed into the fire. A well-known brand was the "Red Comet," which was variously manufactured with other fire-fighting equipment in the Denver, Colorado area by the Red Comet Manufacturing Company from its founding in 1919 until manufacturing operations were closed in the early 1980s.[12]

Niche
Carbon tetrachloride was widely used as a dry cleaning solvent, as a refrigerant, and in lava lamps.[13]
One specialty use of carbon tetrachloride was in stamp collecting, to reveal watermarks on postage stamps without damaging them. A small amount of the liquid was placed on the back of a stamp, sitting in a black glass or obsidian tray. The letters or design of the watermark could then be clearly seen.
Safety
Carbon tetrachloride is one of the most potent hepatotoxins (toxic to the liver), so much so that is widely used in scientific research to evaluate hepatoprotective agents.[7][14]Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system, degenerate the liver[14] and kidneys[15] and prolonged exposure may lead to coma or death.[16] Chronic exposure to carbon tetrachloride can cause liver[17][18] and kidney damage and could result in cancer.[19] See safety data sheets.[20]
The effects of carbon tetrachloride on human health and the environment have been assessed under REACH in 2012 in the context of the substance evaluation by France. Thereafter, further information has been requested from the registrants. Later this decision was reversed.[21]
In 2008, a study of common cleaning products found the presence of carbon tetrachloride in "very high concentrations" (up to 101 mg/m3) as a result of manufacturers' mixing of surfactants or soap with sodium hypochlorite (bleach).[22]
Like many other volatile substances, carbon tetrachloride is prone to misuse by inhalation, due to its possible depressant and/or dissociative effect upon the central nervous system. Use of carbon tetrachloride in this manner presents serious health risks, and may result in toxic effects described above.
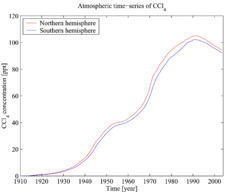
Carbon tetrachloride is also both ozone-depleting[23] and a greenhouse gas.[24] However, since 1992[25] its atmospheric concentrations have been in decline for the reasons described above (see also the atmospheric time-series figure). CCl4 has an atmospheric lifetime of 85 years.[26]
Under high temperatures in air, it forms poisonous phosgene.
References
- 1 2 "Carbon tetrachloride". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0107". National Institute for Occupational Safety and Health (NIOSH).
- ↑ V. Regnault (1839) "Sur les chlorures de carbone CCl et CCl2" (On the chlorides of carbon CCl and CCl2 ), Annales de Chimie et de Physique, vol. 70, pages 104-107. Reprinted in German as: V. Regnault (1839). "Ueber die Chlorverbindungen des Kohlenstoffs, C2Cl2 und CCl2". Annalen der Pharmacie. 30 (3): 350–352. doi:10.1002/jlac.18390300310.
- 1 2 Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Jaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, 2006 Wiley-VCH, Weinheim.doi:10.1002/14356007.a06_233.pub2
- ↑ Carbon tetrachloride
- ↑ F. Brezina, J. Mollin, R. Pastorek, Z. Sindelar. Chemicke tabulky anorganickych sloucenin (Chemical tables of inorganic compounds). SNTL, 1986.
- 1 2 3 Use of Ozone Depleting Substances in Laboratories. TemaNord 516/2003. Archived February 27, 2008, at the Wayback Machine.
- ↑ W. Reusch. "Introduction to Nuclear Magnetic Resonance Spectroscopy". Virtual Textbook of Organic Chemistry. Michigan State University. Archived from the original on August 31, 2006.
- ↑ U.S. Patent 1,010,870, filed April 5, 1910.
- ↑ U.S. Patent 1,105,263, filed Jan 7, 1911.
- ↑ "Pyrene Fire Extinguishers". Vintage Fire Extinguishers. Retrieved 23 December 2009.
- ↑ "Red Comet Manufacturing Company". City of Littleton, CO. Retrieved 30 September 2016.
- ↑ Doherty RE (2000). "A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 1—Historical Background; Carbon Tetrachloride and Tetrachloroethylene". Environmental Forensics. 1 (1): 69–81. doi:10.1006/enfo.2000.0010.
- 1 2 Seifert WF, Bosma A, Brouwer A, et al. (January 1994). "Vitamin A deficiency potentiates carbon tetrachloride-induced liver fibrosis in rats". Hepatology. 19 (1): 193–201. doi:10.1002/hep.1840190129. PMID 8276355.
- ↑ Liu KX, Kato Y, Yamazaki M, Higuchi O, Nakamura T, Sugiyama Y (April 1993). "Decrease in the hepatic clearance of hepatocyte growth factor in carbon tetrachloride-intoxicated rats". Hepatology. 17 (4): 651–60. doi:10.1002/hep.1840170420. PMID 8477970.
- ↑ Recknagel R.O.; Glende E.A.; Dolak J.A.; Waller R.L. (1989). "Mechanism of Carbon-tetrachloride Toxicity". Pharmacology Therapeutics. 43 (43): 139–154. doi:10.1016/0163-7258(89)90050-8.
- ↑ Recknagel RO (June 1967). "Carbon tetrachloride hepatotoxicity". Pharmacol. Rev. 19 (2): 145–208. PMID 4859860.
- ↑ Masuda Y (October 2006). "[Learning toxicology from carbon tetrachloride-induced hepatotoxicity]". Yakugaku Zasshi (in Japanese). 126 (10): 885–99. doi:10.1248/yakushi.126.885. PMID 17016019.
- ↑ Rood AS, McGavran PD, Aanenson JW, Till JE (August 2001). "Stochastic estimates of exposure and cancer risk from carbon tetrachloride released to the air from the rocky flats plant". Risk Anal. 21 (4): 675–95. doi:10.1111/0272-4332.214143. PMID 11726020.
- ↑ Material Safety Data Sheet, Carbon tetrachloride at Fisher Scientific
- ↑ http://echa.europa.eu/information-on-chemicals/evaluation/community-rolling-action-plan/corap-table/-/substance-rev/3080/term
- ↑ Odabasi M (2008). "Halogenated Volatile Organic Compounds from the Use of Chlorine-Bleach-Containing Household Products". Environmental Science & Technology. 42 (5): 1445–51. Bibcode:2008EnST...42.1445O. doi:10.1021/es702355u.
- ↑ Fraser P. (1997). "Chemistry of stratospheric ozone and ozone depletion". Australian Meteorological Magazine. 46 (3): 185–193.
- ↑ Evans WFJ, Puckrin E (1996). "A measurement of the greenhouse radiation associated with carbon tetrachloride (CCl4)". Geophysical Research Letters. 23 (14): 1769–72. Bibcode:1996GeoRL..23.1769E. doi:10.1029/96GL01258.
- ↑ Walker, S. J.; R. F. Weiss & P. K. Salameh (2000). "Reconstructed histories of the annual mean atmospheric mole fractions for the halocarbons CFC-11, CFC-12, CFC-113 and carbon tetrachloride". Journal of Geophysical Research. 105: 14285–96. Bibcode:2000JGR...10514285W. doi:10.1029/1999JC900273.
- ↑ The Atlas of Climate Change (2006) by Kirstin Dow and Thomas E. Downing ISBN 978-0-520-25558-6
External links
- International Chemical Safety Card 0024
- "NIOSH Pocket Guide to Chemical Hazards #0107". National Institute for Occupational Safety and Health (NIOSH).
- "Carbon Tetrachloride (Group 2B)". International Agency for Research on Cancer (IARC) – Summaries & Evaluations. 71: 401. 1999.
- IARC Monograph: "Carbon Tetrachloride"
- Toxicological profile for carbon tetrachloride
- Environmental health criteria for carbon tetrachloride
- Carbon tetrachloride MSDS at Hazardous Chemical Database
- MSDS at Oxford University
- Substance profile at ntp.niehs.nih.gov
- ChemSub Online: Carbon tetrachloride


