Lipid A
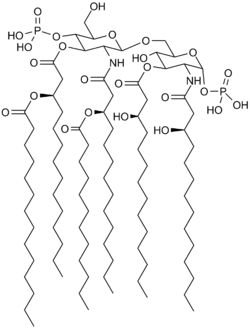
Lipid A is a lipid component of an endotoxin held responsible for the toxicity of gram-negative bacteria. It is the innermost of the three regions of the lipopolysaccharide (LPS), also called endotoxin molecule, and its hydrophobic nature allows it to anchor the LPS to the outer membrane.[2] While its toxic effects can be damaging, the sensing of lipid A by the human immune system may also be critical for the onset of immune responses to gram-negative infection, and for the subsequent successful fight against the infection.[3]
Functions
Many of the immune activating abilities of LPS can be attributed to the lipid A unit. It is a very potent stimulant of the immune system, activating cells (for example, monocytes or macrophages) at picogram per milliliter quantities.
When present in the body at high concentrations during a gram-negative bacterial infection, it may cause shock and death by an "out of control" excessive immune reaction.
Chemical composition
Lipid A consists of two glucosamine (carbohydrate/sugar) units, in an β(1→6) linkage, with attached acyl chains ("fatty acids"), and normally containing one phosphate group on each carbohydrate.[1]
The optimal immune activating lipid A structure is believed to contain 6 acyl chains. Four acyl chains attached directly to the glucosamine sugars are beta hydroxy acyl chains usually between 10 and 16 carbons in length. Two additional acyl chains are often attached to the beta hydroxy group. E. coli lipid A, as an example, typically has four C14 hydroxy acyl chains attached to the sugars and one C14 and one C12 attached to the beta hydroxy groups.[1]
The biosynthetic pathway for Lipid A in E. coli has been determined by the work of Christian R. H. Raetz in the past >32 years.[2] Lipid A structure and effects on eukaryotic cells have been determined and examined, among others, by the groups of Otto Westphal, Chris Galanos, Ernst T. Rietschel and Hajime Takahashi starting already in the 1960s (Gmeiner, Luederitz,Westphal. Eur J Biochem 1969)(Kamio&Takahashi J Biochem 1971)(Luederitz, Galanos et al., J Infect Dis 1973).
Biosynthesis

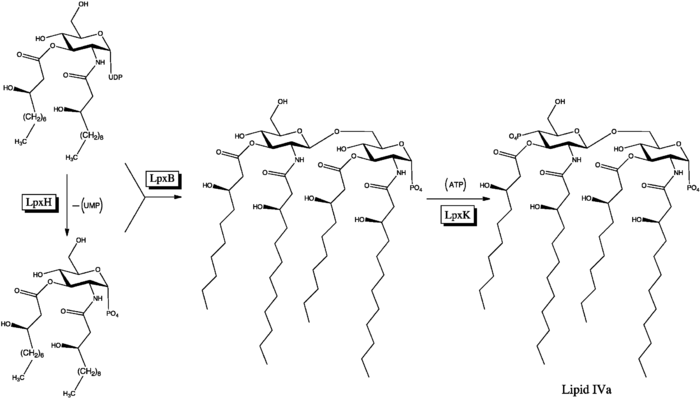
Inhibition and activation of immune response
Lipid A with a reduced number of acyl chains (for example; four) can serve as an inhibitor of immune activation induced by Gram-negative bacteria, and synthetic versions of these inhibitors (Eritoran) were in clinical trials for the prevention of harmful effects caused by gram-negative bacterial infections. However, trials were recently discontinued due to lack of efficacy seen in patients suffering from severe sepsis.[5]
On the other hand, modified versions of lipid A can be used as components of vaccines (adjuvants) to improve their effect.[6] Monophosphorylated lipid A (MPL) is an FDA approved adjuvant that consists of a heterogeneous mixture of lipid A from Salmonella minnesota R595. The major lipid A species present in MPL lacks one of the two phosphate groups and five acyl chains. Other work has shown that the removal of one or two acyl chains from native lipid A can significantly reduce activation of inflammatory responses.[7]
Mechanism of activating cells
Lipid A (and LPS) has been demonstrated to activate cells via Toll-like receptor 4 (TLR4), MD-2 and CD14 on the cell surface.[8][9][10] Consequently, lipid A analogs like eritoran can act as TLR4 antagonists. They are being developed as drugs for the treatment of excessive inflammatory responses to infections with gram-negative bacteria.[11]
See also
References
- 1 2 3 Raetz, Christian R. H.; Guan, Ziqiang; Ingram, Brian O.; Six, David A.; Song, Feng; Wang, Xiaoyuan; Zhao, Jinshi (2009). "Discovery of new biosynthetic pathways: the lipid A story". Journal of Lipid Research: S103–S108.
- 1 2 Raetz C, Whitfield C (2002). "Lipopolysaccharide endotoxins" (abstract). Annu Rev Biochem. 71 (1): 635–700. doi:10.1146/annurev.biochem.71.110601.135414. PMC 2569852
 . PMID 12045108.
. PMID 12045108. - ↑ Tzeng YL, Datta A, Kolli VK, Carlson RW, Stephens DS (May 2002). "Endotoxin of Neisseria meningitidis composed only of intact lipid A: inactivation of the meningococcal 3-deoxy-D-manno-octulosonic acid transferase". J. Bacteriol. 184 (9): 2379–88. doi:10.1128/JB.184.9.2379-2388.2002. PMC 134985
 . PMID 11948150.
. PMID 11948150. - 1 2 King, Jerry D; Kocíncová, Dana; Westman, Erin L; Lam, Joseph S (2009). "Lipopolysaccharide biosynthesis in Pseudomonas aeruginosa". Innate Immunity. 15 (5): 261–312. doi:10.1177/1753425909106436. PMID 19710102.
- ↑ http://jama.jamanetwork.com/article.aspx?articleid=1669798
- ↑ Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, et al. (2010). "Development and Characterization of Synthetic Glucopyranosyl Lipid Adjuvant System as a Vaccine Adjuvant". PLoS ONE. 6 (1): e16333. doi:10.1371/journal.pone.0016333. PMC 3027669
 . PMID 21298114.
. PMID 21298114. - ↑ Needham, Brittany D.; Carroll, Sean M.; Giles, David K.; Georgiou, George; Whiteley, Marvin; Trent, M. Stephen (2013-01-22). "Modulating the innate immune response by combinatorial engineering of endotoxin". Proceedings of the National Academy of Sciences. 110 (4): 1464–1469. doi:10.1073/pnas.1218080110. ISSN 0027-8424. PMC 3557076
 . PMID 23297218.
. PMID 23297218. - ↑ Poltorak, Alexander; He, Xiaolong; Smirnova, Irina; Liu, Mu-Ya; Huffel, Christophe Van; Du, Xin; Birdwell, Dale; Alejos, Erica; Silva, Maria (1998-12-11). "Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene". Science. 282 (5396): 2085–2088. doi:10.1126/science.282.5396.2085. ISSN 0036-8075. PMID 9851930.
- ↑ Park, Beom Seok; Song, Dong Hyun; Kim, Ho Min; Choi, Byong-Seok; Lee, Hayyoung; Lee, Jie-Oh. "The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex". Nature. 458 (7242): 1191–1195. doi:10.1038/nature07830.
- ↑ Beutler, B.; Poltorak, A. (2001-04-01). "The sole gateway to endotoxin response: how LPS was identified as Tlr4, and its role in innate immunity". Drug Metabolism and Disposition: The Biological Fate of Chemicals. 29 (4 Pt 2): 474–478. ISSN 0090-9556. PMID 11259335.
- ↑ Tidswell, M; Tillis, W; Larosa, SP; Lynn, M; Wittek, AE; Kao, R; Wheeler, J; Gogate, J; et al. (2010). "Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis". Critical Care Medicine. 38 (1): 72–83. doi:10.1097/CCM.0b013e3181b07b78. PMID 19661804.
External links
- Lipid A at the US National Library of Medicine Medical Subject Headings (MeSH)
- The Lipid Library - Summary of Lipid A and bacterial polysaccharides
