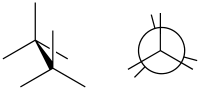
Staggered conformation
For the geometry of staggered rows, see lattice (group).
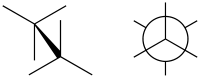
Staggered conformation image right in Newman projection
In organic chemistry, a staggered conformation is a chemical conformation of an ethane-like moiety abcX–Ydef in which the substituents a, b, and c are at the maximum distance from d, e, and f. This requires the torsion angles to be 60°.Eliel, Ernest L.; Wilen, Samuel H. (1994). Stereochemistry of Organic Compounds. Wiley. p. 1207. ISBN 978-0-471-01670-0.
Such a conformation exists in any open chain single chemical bond connecting two sp3-hybridised atoms, and is normally a conformational energy minimum. For some molecules such as those of n-butane, there can be special versions of staggered conformations called gauche and anti; see first Newman projection diagram in Conformational isomerism.
See also
References
This article is issued from Wikipedia - version of the 5/28/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.