Pamela Silver
| Pamela Silver | |
|---|---|
| Nationality | American |
| Institutions | |
| Alma mater | |
Pamela Silver an American cell and systems biologist and a bioengineer. She holds the Elliot T. and Onie H. Adams Professorship of Biochemistry and Systems Biology at Harvard Medical School in the Department of Systems Biology. Silver is one of the founding Core Faculty Members of the Wyss Institute for Biologically Inspired Engineering at Harvard University.
She is one of the founders of the emerging field of Synthetic Biology. She has made contributions to other disciplines including cell and nuclear biology,[1][2][3] systems biology,[4][5] RNA biology,[6][7][8] cancer therapeutics,[9] international policy research, and graduate education. Silver was the first Director of the Harvard University Graduate Program in Systems Biology.
Education and Research
Silver grew up in Atherton, CA where she attended Laurel and Encinal Elementary Schools. During this time, she was a winner of the IBM Math Competition and received special recognition for her early aptitude in science. She attended Menlo Atherton High School and graduated from Castilleja School in Palo Alto. She received her B.A. in Chemistry from the University of California, Santa Cruz and her PhD in Biological Chemistry from the University of California, Los Angeles, where she worked on membrane protein assembly with William Wickner. She did her postdoctoral training with Mark Ptashne at Harvard University where she discovered one of the first nuclear localization sequences.[10]
While in the Ptashne lab, Silver discovered the first nuclear localization sequence (NLS) in the yeast GAL4 protein.[11] She continued to study the mechanism of nuclear localization in her own lab as an Assistant Professor at Princeton University. During this time, she characterized the receptor for NLSs and discovered the first eukaryotic DnaJ chaperone.[12]
Silver continued in the area of Cell Biology upon moving to the Dana Farber Cancer Institute to hold the Claudia Adams Barr Investigatorship and to become Associate Professor of Biological Chemistry and Molecular Pharmacology at Harvard Medical School and Dana-Farber. During this time, she was among the first to follow GFP-tagged proteins in living cells.[13] In addition, she initiated early studies in systems biology to examine interactions within the nucleus on a whole genome scale.[14] Together with Bill Sellers, she discovered molecules that block nuclear export[15] and formed the basis for a publicly traded company Karyopharm Therapeutics. She was promoted in 1997 to Professor of Biological Chemistry and Molecular Pharmacology at Harvard Medical School and Dana-Farber.
In 2004, Silver moved to the newly formed Department of Systems Biology at Harvard Medical School as a Professor. Around this time, she engaged with the Synthetic Biology Working Group at MIT and made the decision to move her research group into Synthetic Biology. Since then, she has developed numerous genetic circuits in all types of cells,[16] engineered carbon fixation,[17] and developed new therapeutic proteins and biofuel precursors.[18][19] She was the first to observe the motion of the carbon fixing organelles in photosynthetic bacteria.[20] During this time, she has also been the Director of an ARPA-E (DOE) project on electrofuels.
Contributions to Science
Synthetic Biology
Silver seeks to re-program both eukaryotic and prokaryotic cells. Some of Silver’s work in this area includes the engineering of: mammalian cells to remember and report past exposures to drugs and radiation,[21][22][23] robust computational circuits in embryonic stem cells and bacteria,[24] and synthetic switches to moderate gene silencing with the integration of novel therapeutic proteins.[25][26] Silver has engineered natural gut bacteria that can pass through an animal’s digestive tract and report whether an animal has been exposed to a drug.[27] Given that gut microbiota plays a key role in maintaining the health of all mammals and is a potential site for diagnostic and therapeutic intervention, Silver’s work sets the stage for the development of novel therapies for use in both humans and in the livestock industry.
Carbon fixation and sustainability
Silver has characterized the carboxysome – the major carbon-fixing structure in cyanobacteria – to enhance photosynthetic efficiency[28] and carbon fixation.[29] She has also engineered cyanobacteria to more efficiently cycle carbon into high-value commodities and has shown that these bacteria can form sustainable consortia.[30]
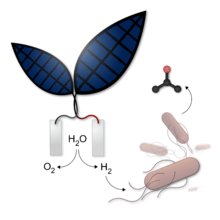
Silver collaborated with Daniel Nocera at Harvard University to develop a device, called the "Bionic Leaf", that converts solar energy into fuel through a hybrid water-splitting catalyst system that leverages metabolically-engineered bacteria. The device is as efficient at producing hydrogen as natural plants and can be easily modified to reach higher efficiency of conversion and storage of solar energy.[31]
Gene regulation
Silver discovered a strong correlation between nuclear transport and gene regulation – she identified the first arginine methyltransferase, which plays a role in chromatin function and is important to the movement of RNA binding proteins between the nucleus and cytoplasm of cells. She also discovered previously unknown variations among ribosomes that led her to propose a unique specificity for the matching between ribosomes and the subsequent translation of mRNAs. Silver’s finding has several implications towards our understanding of how gene regulation impacts disease development, such as cancer.[32]
Awards
Silver has been the recipient of an NSF Presidential Young Investigator Award, a Basil O’Connor Research Scholar of the March of Dimes, an Established Investigator of the American Heart Association, the NIH Directors Lecture, and NIH MERIT award, Innovation award at BIO, a Fellow of the Radcliffe Institute for Advanced Study, the Elliot T. and Onie H. Adams Professorship at Harvard Medical School and named the Top 20 Global Synthetic Biology Influencers. She sits on numerous advisory boards and has presented to members of the US Congress.
Silver was awarded the BBS Mentoring Award for Graduate Education at Harvard Medical School. She is also one of the founders of the International Genetically Engineered Machines competition (iGEM) and currently sits of the Board of iGEM.org. Silver founded and was the first Director of the Harvard University Graduate Program in Systems Biology. Silver is a strong advocate for women in science.
External links
- The Silver Lab
- Silver profile page, Wyss Institute
- Department of Systems Biology at Harvard
- Systems Biology PhD Program
- iGEM
References
- ↑ Jason A Kahana; Bruce J Schnapp; Pamela A Silver (October 10, 1995). "Kinetics of spindle pole body separation in budding yeast". Proceedings of the National Academy of Sciences. 92 (21): 9707–9711. doi:10.1073/pnas.92.21.9707. PMID 7568202. Retrieved 6 May 2015.
- ↑ PA Silver; LP Keegan; M Ptashine (October 1, 1984). "Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization". Proceedings of the National Academy of Sciences. 81 (19): 5951–5. doi:10.1073/pnas.81.19.5951. PMID 6091123. Retrieved 6 May 2015.
- ↑ Casolari, J.M.; Brown, C.R.; Komili, S.; West, J.; Hieronymus, H. & Silver, P.A. (May 14, 2004). "Genome-wide localization of the nuclear transport machinery reveals coupling of transcriptional status and nuclear organization". Cell. 117 (4): 427–439. doi:10.1016/s0092-8674(04)00448-9. PMID 15137937.
- ↑ Jason S Carroll; X Shirley Liu; Alexander S Brodsky; Wei Li; Clifford A Meyer; Anna J Szary; Jerome Eeckhoute; Wenlin Shao; Eli V Hestermann; Timothy R Geistlinger; Edward A Fox; Pamela A Silver; Myles Brown (July 15, 2005). "Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forehead protein FoxA1". Cell. 122 (1): 33–43. doi:10.1016/j.cell.2005.05.008. PMID 16009131. Retrieved 6 May 2015.
- ↑ Haley Hieronymus; Pamela A Silver (February 1, 2003). "Genome-wide analysis of RNA-protein interactions illustrates specificity of the mRNA export machinery". Nature Genetics. 33 (2): 155–161. doi:10.1038/ng1080. Retrieved 6 May 2015.
- ↑ Michael J Moore; Qingqing Wang; Caleb J Kennedy; Pamela A Silver (August 20, 2010). "An alternative splicing network links cell-cycle control to apoptosis". Cell. 142 (4): 625–636. doi:10.1016/j.cell.2010.07.019.
- ↑ Elisa C Shen; Michael F Henry; Valerie H Weiss; Sandro R Valentini; Pamela A Silver; Margaret S Lee (March 1, 1998). "Arginine methylation facilitates the nuclear export of hnRNP proteins". Genes & Development. 12 (5): 679–691. doi:10.1101/gad.12.5.679. PMID 9499403.
- ↑ Margaret S Lee; Michael Henry; Pamela A Silver (May 15, 1996). "A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export". Genes & Development. 10 (10): 1233–1246. doi:10.1101/gad.10.10.1233.
- ↑ Tweeny R Kau; Frank Schroeder; Shivapriya Ramaswamy; Cheryl L Wojciechowski; Jean J Zhao; Thomas M Roberts; Jon Clardy; William R Sellers; Pamela A Silver (December 31, 2003). "A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells". Cancer Cell. 4 (6): 463–476. doi:10.1016/S1535-6108(03)00303-9.
- ↑ Silver, P.; Keegan, L. & Ptashne, M. (1984). "The amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization". Proc. Natl. Acad. Sci. USA. 81: 5951–5. doi:10.1073/pnas.81.19.5951. PMID 6091123.
- ↑ Silver, P.; Chiang, A. & Sadler, I. (1988). "Mutations affecting localization and production of a yeast nuclear protein". Genes & Development. 2: 707–17. doi:10.1101/gad.2.6.707.
- ↑ Blumberg, H. & Silver, P. (1991). "SCJ1, a DNAJ homologue that alters protein sorting in yeast". Nature. 349: 627–30. doi:10.1038/349627a0.
- ↑ Kahana, J.; Schnapp, B. & Silver, P. (1995). "Kinetics of spindle pole body separation in budding yeast". Proc. Natl. Acad. Sci. 92: 9707–9711. doi:10.1073/pnas.92.21.9707. PMID 7568202.
- ↑ Casolari, J.; Brown, CR; Komili, S.; West, J.; Hieronymus, H. & Silver, PA. (2004). "Genome-wide localization of the nuclear transport machinery reveals coupling of transcriptional status and nuclear organization". Cell. 117: 427–439. doi:10.1016/s0092-8674(04)00448-9. PMID 15137937.
- ↑ Kau, TR; Schroeder, F; Wojciechowski, C.; Zhou, JJ; Roberts, T.; Clardy, J; Sellers, W & Silver, PA. (2003). "A chemical genetic screen for inhibitors of regulated export of a Forkhead transcription factor in tumor cells". Cancer Cell. 4: 463–476. doi:10.1016/s1535-6108(03)00303-9.
- ↑ Smolke CD & Silver PA (2011). "Informing biological design by integration of systems and synthetic biology". Cell. 144 (6): 855–9. doi:10.1016/j.cell.2011.02.020.
- ↑ Bonacci W, Afonso B, Silver PA, Savage DF (2012). "Modularity of a carbon-fixing proteinorganelle". Proc. Natl. Acad. Sci. USA. 109 (2): 478–83. doi:10.1073/pnas.1108557109.
- ↑ Delebecque CJ, Lindner AB, Silver PA & Aldaye FA (2011). "Organization of intracellular reactions with rationally designed RNA assemblies.". Science. 333: 470–4. doi:10.1126/science.1206938. PMID 21700839.
- ↑ Torella J, Ford T, Silver PA (2013). "Tailored fatty acid synthesis via dynamic control of fatty acid elongation". Proc. Natl. Acad. Sci. USA. 110 (28): 11290–5. doi:10.1073/pnas.1307129110.
- ↑ Savage D, Afonso B, Silver PA (2010). "Spatially ordered dynamics of the bacterial carbon fixation machinery". Science. 327: 1258–61. doi:10.1126/science.1186090.
- ↑ Ajo-Franklin, CM; Drubin, DA; Eskin, J.; Gee, E.; Landgraf, D.; Philips, I. & Silver, PA. (September 15, 2007). "Rational design of memory in eukaryotic cells". Genes & Development. 21 (18): 2271–2276. doi:10.1101/gad.1586107. PMID 17875664.
- ↑ Burrill D & Silver PA (2011). "Synthetic circuit identifies sub-populations with sustained memory of DNA damage". Genes & Development. 25: 434–439. doi:10.1101/gad.1994911. PMC 3049284
 . PMID 21363961.
. PMID 21363961. - ↑ Burrill DR, Inniss MC, Boyle PM & Silver PA (July 1, 2012). "Synthetic memory circuits for tracking human cell fate". Genes & Development. 26 (13): 1486–1497. doi:10.1101/gad.189035.112. PMID 22751502.
- ↑ Robinson-Mosher A, Chen JH, Way J, Silver PA (November 18, 2014). "Designing cell-targeted therapeutic proteins reveals the interplay between domain connectivity and cell binding". Biophysical Journal. 107 (10): 2456–2466. doi:10.1016/j.bpj.2014.10.007. PMID 25418314.
- ↑ Haynes KA, Silver PA (August 5, 2011). "Synthetic reversal of epigenetic silencing". Journal of Biological Chemistry. 286 (31): 27176–27182. doi:10.1074/jbc.C111.229567. PMID 21669865.
- ↑ Alexander A. Green; Pamela A. Silver; James J. Collins & Peng Yin (November 6, 2014). "Toehold Switches: De-Novo-Designed Regulators of Gene Expression" (PDF). Cell. 159: 1–15. doi:10.1016/j.cell.2014.10.002. Retrieved 7 May 2015.
- ↑ Kotula JW, Kerns SJ, Shaket LA, Siraj L, Collins JJ, Way JC, SIlver PA (April 1, 2014). "Programmable bacteria detect and record an environmental signal in the mammalian gut". Proceedings of the National Academy of Sciences. 111 (13): 4838–4843. doi:10.1073/pnas.1321321111. PMID 24639514.
- ↑ Ducat DC, Avelar-Rivas JA, Way JC, Silver PA (April 2012). "Rerouting carbon flux to enhance photosynthetic productivity". Applied and Environmental Microbiology. 78 (8): 2660–2668. doi:10.1128/AEM.07901-11. PMID 22307292.
- ↑ Ducat DC, Silver PA (August 2012). "Improving carbon pathways". Current Opinion in Chemical Biology. 16 (3-4): 337–344. doi:10.1016/j.cbpa.2012.05.002. PMID 22647231.
- ↑ Polka J, Silver PA (December 1, 2013). "Building synthetic cellular organization". Molecular Biology of the Cell. 24 (23): 3585–3587. doi:10.1091/mbc.E13-03-0155. PMC 3842987
 . PMID 24288075.
. PMID 24288075. - ↑ Torella JP, Gagliardi CJ, Chen JS, Bediako DK, Colon B, Way JC, SIlver PA, Nocera DG (February 24, 2015). "Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system". Proceedings of the National Academy of Sciences. 112 (8): 2337–2342. doi:10.1073/pnas.1424872112. PMID 25675518.
- ↑ Yu MC, Lamming DW, Eskin JA, Sinclair DA & Silver PA (December 1, 2006). "The role of arginine methylation in formation of silent chromatin". Genes & Development. 20 (23): 3249–3254. doi:10.1101/gad.1495206. PMID 17158743.