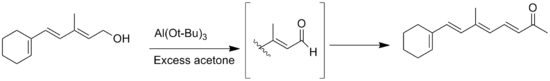Oppenauer oxidation
Oppenauer oxidation, named after Rupert Viktor Oppenauer,[1] is a gentle method for selectively oxidizing secondary alcohols to ketones.

The reaction is the opposite of Meerwein–Ponndorf–Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone. This shifts the equilibrium toward the product side.
The oxidation is highly selective for secondary alcohols and does not oxidize other sensitive functional groups such as amines and sulfides.[2] Though primary alcohols can be oxidized under Oppenauer conditions, primary alcohols are seldom oxidized by this method due to the competing aldol condensation of aldehyde products. The Oppenauer oxidation is still used for the oxidation of acid labile substrates. The method has been largely displaced by oxidation methods based on chromates (e.g. pyridinium chlorochromate) or dimethyl sulfoxide (e.g. Swern oxidation) or Dess–Martin oxidation due to its use of relatively mild and non-toxic reagents (e.g. the reaction is run in acetone/benzene mixtures). The Oppenauer oxidation is commonly used in various industrial processes such as the synthesis of steroids, hormones, alkaloids, terpenes, etc.
Mechanism
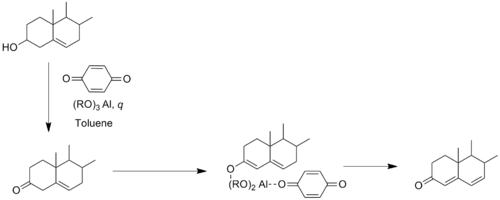
In the first step of the mechanism, the alcohol (1) coordinates to the aluminium to form a complex (3), which then, in the second step, gets deprotonated by an alkoxide ion (4) to generate an alkoxide intermediate (5). In the third step, both the oxidant acetone (7) and the substrate alcohol are bound to the aluminium. The acetone is coordinated to the aluminium which activates it for the hydride transfer from the alkoxide. The aluminium-catalyzed hydride shift from the α-carbon of the alcohol to the carbonyl carbon of acetone proceeds over a six-membered transition state (8). The desired ketone (9) is formed after the hydride transfer.[3]
Advantages
An advantage of the Oppenauer oxidation is its use of relatively inexpensive and non-toxic reagents. Reaction conditions are mild and gentle since the substrates are generally heated in acetone/benzene mixtures. Another advantage of the Oppenauer oxidation which makes it unique to other oxidation methods such as pyridinium chlorochromate (PCC) and Dess–Martin periodinane is that secondary alcohols are oxidized much faster than primary alcohols, thus chemoselectivity can be achieved. Furthermore, there is no over oxidation of aldehydes to carboxylic acids as opposed to another oxidation methods such the Jones oxidation.[3]
Modifications
Wettstein-Oppenauer reaction
In the Wettstein-Oppenauer reaction, discovered by Wettstein in 1945, Δ 5–3β-hydroxy steroids are oxidized to Δ 4,6-3-ketosteroids with benzoquinone as the hydrogen acceptor. This reaction is useful in that it affords a one-step preparation of Δ 4,6-3-ketosteroids.[4]
Woodward modification
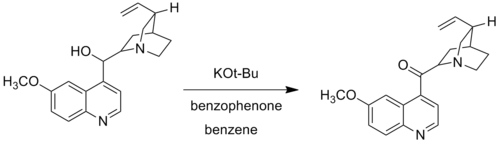
In the Woodward modification, Woodward substituted potassium tert-butoxide for the aluminium alkoxide. The Woodward modification of the Oppenauer oxidation is used when certain alcohol groups do not oxidize under the standard Oppenauer reaction conditions. For example, Wooward used potassium tert-butoxide and benzophenone for the oxidation of quinine to quininone, as the traditional aluminium catalytic system failed to oxidize quinine due to the complex formed by coordination of the Lewis-basic nitrogen to the aluminium centre.[5]
Other modifications
Several modified aluminium alkoxide catalysts have been also reported. For example, a highly active aluminium catalyst was reported by Maruoka and co-workers which was utilized in the oxidation of carveol to carvone (a member of a family of chemicals called terpenoids) in excellent yield (94%).[6]
In another modification [7] the catalyst is trimethylaluminium and the aldehyde 3-nitrobenzaldehyde is used as the oxidant, for example, in the oxidation of isoborneol to camphor.
Synthetic applications
The Oppenauer oxidation is used to prepare analgesics in the pharmaceutical industry such as morphine and codeine. For instance, codeinone is prepared by the Oppenauer oxidation of codeine.[8]
The Oppenauer oxidation is also used to synthesize hormones. Progesterone is prepared by the Oppenauer oxidation of pregnenolone.[9]
A slight variation of the Oppenauer oxidation is also used to synthesize steroid derivatives. For example, an efficient catalytic version of the Oppenauer oxidation which employs a ruthenium catalyst has been developed for the oxidation of 5-unsaturated 3β-hydroxy steroids to the corresponding 4-en-3-one derivative.[10]
The Oppenauer oxidation is also used in the synthesis of lactones from 1,4 and 1,5 diols.[11]
Side reactions
A common side-reaction of the Oppenauer oxidation is the base-catalyzed aldol condensation of aldehyde products, which have α-hydrogens to form either ß-hydroxy aldehydes or α, ß-unsaturated aldehydes.[12]
Another side reaction is the Tischenko reaction of aldehyde products with no α-hydrogen, but this can be prevented by use of anhydrous solvents.[3] Another general side reaction is the migration of the double bond during the oxidation of allylic alcohol substrates.[13]
References
- ↑ Oppenauer, R. V. (1937) Recl. Trav. Chim. Pays-Bas, 56, 137–144.
- ↑ Otvos, L.; Gruber, L.; Meisel-Agoston, J. (1965). "The Meerwein-Ponndorf-Verley-Oppenauer. Investigation of the reaction mechanism with radiocarbon. Racemization of secondary alcohols". Acta Chim. Acad. Sci. Hung. 43: 149–153.
- 1 2 3 Corey, E.J; Nicolaou, K.C. (2005). Strategic Applications of Named Reactions in Organic Synthesis. Elsevier. ISBN 978-7-03-019190-8.
- ↑ Mandell, L. (1955). "The Mechanism of the Wettstein-Oppenauer Oxidation". J. Am. Chem. Soc. 78 (13): 3199–3201. doi:10.1021/ja01594a061.
- ↑ Woodward, R. B.; Wendler, N. L.; Brutschy, F. J. (1945). "Quininone1". J. Am. Chem. Soc. 67 (9): 1425. doi:10.1021/ja01225a001.
- ↑ Ooi, T; Otsuka, H; Miura, T; Ichikawa, H; Maruoka, K (2002). "Practical Oppenauer (OPP) oxidation of alcohols with a modified aluminum catalyst". Organic Letters. 4 (16): 2669–72. doi:10.1021/ol020094c. PMID 12153205.
- ↑ Graves, C. R.; Zeng, B. S.; Nguyen, S. T. (2006). "Efficient and Selective Al-Catalyzed Alcohol Oxidation via Oppenauer Chemistry". Journal of the American Chemical Society. 128 (39): 12596–7. doi:10.1021/ja063842s. PMID 17002323.
- ↑ Stéphane Caron; Robert W. Dugger; Sally Gut Ruggeri; John A. Ragan & David H. Brown Ripin (2006). "Large-Scale Oxidations in the Pharmaceutical Industry". Chem. Rev. 106 (7): 2943–89. doi:10.1021/cr040679f. PMID 16836305.
- ↑ Dewick, P (2001). Medicinal Natural Products: A Biosynthetic Approach (PDF) (2nd ed.). Wiley & Sons. p. 243. ISBN 0471496405.
- ↑ Almeida, Maria L.S.; Kočovský, Paval; Bäckvall, Jan-E. (1996). "Ruthenium-Catalyzed Oppenauer-Type Oxidation of 3β-Hydroxy Steroids. A Highly Efficient Entry into the Steroidal Hormones with 4-En-3-one Functionality". J. Org. Chem. 61 (19): 6587–6590. doi:10.1021/jo960361q.
- ↑ Eignerova, L.; Kasal, A. (1976). Collect. Czech. Chem. Commun. 41: 1056
- ↑ Milas, N. A.; Grossi, F. X.; Penner, S. E.; Kahn, S. (1948). "The Synthesis of 1-[cyclohexen-1'-yl]-3-Methyl-1,3,5-Octatrien-7-One (C15Ketone)1". Journal of the American Chemical Society. 70 (3): 1292. doi:10.1021/ja01183a522.
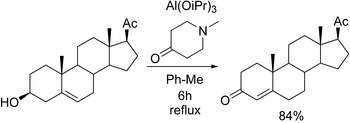
- ↑ Reich, R.; Keana, J. F. W. (1972). "Oppenauer Oxidations Using 1-Methyl-4-Piperidone as the Hydride Acceptor". Synthetic Communications. 2 (5): 323. doi:10.1080/00397917208061988.
- ↑ Reich, Richard; Keana, John F. W. (1972). "Oppenauer Oxidations Using 1-Methyl-4-Piperidone as the Hydride Acceptor". Synth Commun. 2 (5): 323–325. doi:10.1080/00397917208061988.