Molecular logic gate
| Part of a series of articles on |
| Nanoelectronics |
|---|
| Single-molecule electronics |
| Solid state nanoelectronics |
| Related approaches |
|
A molecular logic gate is a molecule that performs a logical operation based on one or more physical or chemical inputs and a single output. The field has advanced from simple logic systems based on a single chemical or physical input to molecules capable of combinatorial and sequential operations such as arithmetic operations i.e. moleculators and memory storage algorithms.
For logic gates with a single input, there are four possible output patterns. When the input is 0, the output can be either a 0 or 1. When the input is 1, the output can again be 0 or 1. The four output bit patterns that can arise corresponds to a specific logic type: PASS 0, YES, NOT and PASS 1. PASS 0 always outputs 0, whatever the input. PASS 1 always outputs 1, whatever the input. YES outputs a 1 when the input is 1 and NOT is the inverse YES - it outputs a 0 when the input is 1. An example of a YES logic gate is the molecular structure shown below. A ‘1’ output is given only when sodium ions are present in solution (‘1’ input).

Molecular logic gates work with input signals based on chemical processes and with output signals based on spectroscopy. One of the earlier water solution-based systems exploits the chemical behavior of compounds A and B in scheme 1 .

Compound A is a push-pull olefin with the top receptor containing four carboxylic acid anion groups (and non-disclosed counter cations) capable of binding to calcium. The bottom part is a quinoline molecule which is a receptor for hydrogen ions. The logic gate operates as follows. Without any chemical input of Ca2+ or H+, the chromophore shows a maximum absorbance in UV/VIS spectroscopy at 390 nm. When calcium is introduced a blue shift takes place and the absorbance at 390 nm decreases. Likewise addition of protons causes a red shift and when both cations are in the water the net result is absorption at the original 390 nm. This system represents a XNOR logic gate in absorption and a XOR logic gate in transmittance.
In compound B the bottom section now contains a tertiary amino group also capable of binding to protons. In this system fluorescence only takes place when both cations available. The presence of both cations hinders photoinduced electron transfer (PET) allowing compound B to fluoresce. In the absence of both or either ion, fluorescence is quenched by PET, which involves an electron transfer from either the nitrogen atom or the oxygen atoms, or both to the anthracenyl group. When both receptors are bound to calcium ions and protons respectively, both PET channels are shut off. The overall result of Compound B is AND logic, since an output of “1” (fluorescence) occurs only when both Ca2+ and H+ are present in solution, that is, have values as “1”. With both systems run in parallel and with monitoring of transmittance for system A and fluorescence for system B the result is a half-adder capable of reproducing the equation 1+1=2.
In a modification of system B not two but three chemical inputs are simultaneously processed in an AND logic gate . An enhanced fluorescence signal is observed only in the presence of excess protons, zinc and sodium ions through interactions with their respective amine, phenyldiaminocarboxylate and crown ether receptors. The processing mode operates similarly as discussed above - fluorescence is observed due to the prevention of competing photoinduced electron transfer reactions from the receptors to the excited anthracene fluorophore. The absence of one, two or all three ion inputs results in a low fluorescence output. Each receptor is selective for its specific ion as an increase in the concentration of the other ions does not yield a high fluorescence. The specific concentration threshold of each input must be reached to achieve a fluorescent output in accordance with combinatorial AND logic. This prototype could potentially be extended to point-of-care medical diagnostics application for disease screening in the future.

In a similar set-up, the molecular logic gate illustrated below demonstrates the advancement from redox-fluorescent switches to multi-input logic gates with an electrochemical switch. This two-input AND logic gate incorporates a tertiary amine proton receptor and a tetrathiafulvelene redox donor. These groups, when attached to anthracene can simultaneously process information concerning acid concentration and oxidizing ability of the solution.

The INHIBIT logic gate illustrated below as provided by Gunnlaugsson et al. incorporates a Tb3+ ion in a chelate complex. This two-input logic gate is the first of its kind and displays non-commutative behaviour with chemical inputs and a phosphorescence output. Whenever dioxygen (input 1) is present, the system is quenched and no phosphorescence is observed (output 0). The second input, H+, must also be present for an output "1" to be observed. This is understood from a two-input INHIBIT truth table.

In another XOR logic gate system the chemistry is based on the pseudorotaxane depicted in scheme 3. In organic solution the electron deficient diazapyrenium salt (rod) and the electron rich 2,3-dioxynaphthalene units of the crown ether (ring) self-assemble by formation of a charge transfer complex.
An added tertiary amine like tributylamine forms a 1:2 adduct with the diazapyrene and the complex gets dethreaded. This process is accompanied by an increase in emission intensity at 343 nm resulting from freed crown ether. Added trifluoromethanesulfonic acid reacts with the amine and the process is reverted. Excess acid locks the crown ether by protonation and again the complex is dethreaded.

A full adder system based on fluorescein is able to compute 1+1+1=3.
Molecular sequential logic is exemplified by D. Margulies et al., where they demonstrate a molecular keypad lock resembling the processing capabilities of an electronic security device which is equivalent to incorporates several interconnected AND logic gates in parallel. The molecule mimics an electronic keypad of an automated teller machine (ATM). The output signals are dependent not only on the combination of inputs but also on the correct order of inputs: in other words the correct password must be entered. The molecule was designed using pyrene and fluorescein fluorophores connected by a siderophore, which binds to Fe(III), and the acidic of the solution changes the fluorescence properties of the fluorescein fluorophore.
Further development in this field might also see molecular logic gates replace semiconductors in the IT industry. Such molecular systems can theoretically overcome the problems arising when semiconductors approach nano-dimensions. Molecular logic gates are more versatile than their silicon counterparts, with phenomena such as superposed logic unavailable to semiconductor electronics. Dry molecular gates such as the one demonstrated by Avouris and colleagues prove to be possible substitutes for semiconductor devices due to their small size, similar infrastructure and data processing abilities. Avouris revealed a NOT logic gate composed of a bundle of carbon nanotubes. The nanotubes are doped differently in adjoining regions creating two complementary field effect transistors. The bundle operates as a NOT logic gate only when satisfactory conditions are met.
New potential applications of chemical logic gates continue to be explored. A recent study illustrates the application of a logic gate for photodynamic therapy. A bodipy dye attached to a crown-ether and two pyridyl groups separated by spacers (as shown below) works according to an AND logic gate. The molecule works as a photodynamic agent upon irradiation at 660 nm under conditions of relatively high sodium and proton ion concentrations by converting triplet oxygen to cytotoxic singlet oxygen. This prototypical example would take advantage of the higher sodium levels and lower pH in tumor tissue compared to the levels in normal cells. When these two cancer-related cellular parameters are satisfied, a change is observed in the absorbance spectrum. This technique could be useful for the treatment of malignant tumors as it is non-invasive and specific.

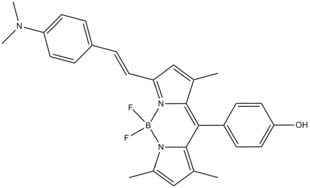
A molecular logic gate can processes modulators much like the set up seen in de Silva’s ‘Proof-of-principle’ but incorporating different logic gates on the same molecule. Such a function is called integrated logic and is exemplified by the BODIPY-based, half-subtractor logic gate illustrated by A. Coskun, E. U. Akkaya and their colleagues (as shown below). When monitored at two different wavelengths, 565 and 660 nm, XOR and INHIBIT logic gates are obtained at the respective wavelengths. Optical studies of this compound in THF reveal an absorbance peak at 565 nm and an emission peak at 660 nm. Addition of an acid results in a hypsochromic shift of both peaks as protonation of the tertiary amine results in an internal charge transfer. The colour of the emission observed is yellow. Upon addition of a strong base, the phenolic hydroxyl group is rendered deprotonated, resulting in a photoinduced electron transfer, which in turn renders the molecule non-emissive. Upon addition of both an acid and a base, the emission of the molecule is observed as red, as the tertiary amine would not be protonated while the hydroxyl group would remain protonated resulting in the absence of both PET and ICT. Due to the great difference in emission intensity, this single molecule is capable of carrying out an arithmetic operation; subtraction at a nanoscale level.
See also
References
- ^ A. Prasanna de Silva and Nathan D. McClenaghan. Proof-of-Principle of Molecular-Scale Arithmetic J. Am. Chem. Soc. 2000, 122, 16, 3965–3966. Abstract
- ^ David C. Magri, Gareth J. Brown, Gareth D. McClean and A. Prasanna de Silva. Communicating Chemical Congregation: A Molecular AND Logic Gate with Three Chemical Inputs as a "Lab-on-a-Molecule" Prototype J. Am. Chem. Soc. 2006, 128, 4950–4951. (Communication) Abstract
- ^ David C. Magri. A fluorescent AND logic gate driven by electrons and protons. New J. Chem. 2009, 33, 457–461.
- ^ T. Gunnlaugsson, D.A. MacDonail and D. Parker, Chem. Commun. 2000, 93.
- ^ Alberto Credi, Vincenzo Balzani, Steven J. Langford, and J. Fraser Stoddart. Logic Operations at the Molecular Level. An XOR Gate Based on a Molecular Machine J. Am. Chem. Soc. 1997,119, 2679–2681.(Article) Abstract
- ^ David Margulies, Galina Melman, and Abraham Shanzer. A Molecular Full-Adder and Full-Subtractor, an Additional Step toward a Moleculator J. Am. Chem. Soc. 2006, 128, 4865–4871. (Article) doi:10.1021/ja058564w
- ^ David Margulies, Galina Melman, and Abraham Shanzer. A molecular keypad lock: A photochemical device capable of authorizing password entries. J. Am. Chem. Soc. 2007, 129, 347–354.
- ^ S. Oslem and E.U. Akkaya. Thinking outside the silicon box: molecular AND logic as an additional layer of selectivity in singlet oxygen generation for photodynamic therapy. J. Am. Chem. Soc. 2009, 131, 48–49.
- ^ A. Coskun, E. Deniz and E.U. Akkaya. Effective PET and ICT switching of boradiazaindacene emission: A unimolecular, emission-mode, molecular half-subtractor with reconfigurable logic gates. Org. Lett. 2005 5187–5189.
External links
- A Molecular Photoionic AND Gate Based on Fluorescent Signaling
- The 3rd International Conference on Molecular Sensors & Molecular Logic Gates (MSMLG) was held on July 8–11, 2012 at Korea University in Seoul, Korea.
