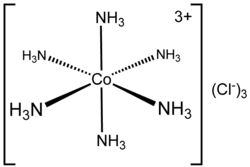
Hexamminecobalt(III) chloride
 | |
Chloride.jpg) | |
| Names | |
|---|---|
| IUPAC name
Hexaamminecobalt(III) chloride | |
| Other names
Cobalt hexammine chloride, hexaamminecobalt(III) chloride | |
| Identifiers | |
| 10534-89-1 | |
| ECHA InfoCard | 100.030.991 |
| UNII | 240056WZHT |
| Properties | |
| H18N6Cl3Co | |
| Molar mass | 267.48 g/mol |
| Appearance | yellow or orange crystals |
| Density | 1.71 g/cm3, |
| Melting point | decomposes |
| 0.26 M (20 °C) tribromide: 0.04 M (18 °C) | |
| Solubility | soluble in NH3 |
| Structure | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | poison |
| R-phrases | 36/37/38 |
| S-phrases | none |
| Related compounds | |
| Other anions |
[Co(NH3)6]Br3 [Co(NH3)6](OAc)3 |
| Other cations |
[Cr(NH3)6]Cl3 [Ni(NH3)6]Cl2 |
| Related compounds |
[Co(H2NCH2CH2NH2)3]Cl3 [Co(NH3)5(H2O)]Cl3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. This coordination compound is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. This salt consists of [Co(NH3)6]3+ trications with three Cl− anions. The term ammine refers to ammonia in its metal complexes, and the prefix hexa- (Greek: six) indicates that there are six ammonia molecules per cation.
Originally this compound was described as a "luteo" (Latin: yellow) complex, but this name has been discarded as modern chemistry considers color less important than molecular structure. Other similar complexes also had color names, such as purpureo (Latin: purple) for a pentammine complex, and praseo (Greek: green) and violeo (Latin: violet) for two isomeric tetrammine complexes. [1]
Properties and structure
[Co(NH3)6]3+ is diamagnetic, with a low-spin octahedral Co(III) center. The cation obeys the 18-electron rule and is considered to be a classic example of an exchange inert metal complex. As a manifestation of its inertness, [Co(NH3)6]Cl3 can be recrystallized unchanged from concentrated hydrochloric acid: the NH3 is so tightly bound to the Co(III) centers that it does not dissociate to allow its protonation. In contrast, labile metal ammine complexes, such as [Ni(NH3)6]Cl2, react rapidly with acids reflecting the lability of the Ni(II)–NH3 bonds. Upon heating, hexamminecobalt(III) begins to lose some of its ammine ligands, eventually producing a stronger oxidant.
The chloride ions in [Co(NH3)6]Cl3 can be exchanged with a variety of other anions such as nitrate, bromide, and iodide to afford the corresponding [Co(NH3)6]X3 derivative. Such salts are bright yellow and display varying degrees of water solubility.
Preparation
Since CoCl3 is not available, [Co(NH3)6]Cl3 is prepared from cobalt(II) chloride. The latter is treated with ammonia and ammonium chloride followed by oxidation. Oxidants include hydrogen peroxide or oxygen in the presence of charcoal catalyst.[2] This salt appears to have been first reported by Fremy.[3]
The acetate salt can be prepared by aerobic oxidation of cobalt(II) acetate, ammonium acetate, and ammonia in methanol.[4] The acetate salt is highly water-soluble to the level of 1.9 M (20 °C), versus 0.26 M for the trichloride.
Uses
[Co(NH3)6]3+ is a component of some structural biology methods (especially for DNA or RNA, where positive ions stabilize tertiary structure of the phosphate backbone), to help solve their structures by X-ray crystallography[5] or by nuclear magnetic resonance.[6] In the biological system, the counterions would more probably be Mg2+, but the heavy atoms of cobalt (or sometimes iridium, as in PDB file 2GIS) provide anomalous scattering to solve the phase problem and produce an electron-density map of the structure.[7]
References
- ↑ Huheey, James E. (1983). Inorganic Chemistry (3rd ed.). p. 360.
- ↑ Bjerrum, J.; McReynolds, J. P. (1946). "Hexamminecobalt(III) Salts". Inorg. Synth. 2: 216–221. doi:10.1002/9780470132333.ch69.
- ↑ Fremy, M. E. (1852). "Recherches sur le cobalt". Ann. Chim. Phys. 35: 257–312.
- ↑ Lindholm,, R. D.; Bause, Daniel E. (1978). "Hexamminecobalt(III) Salts". Inorg. Synth. 18: 67–69. doi:10.1002/9780470132494.ch14.
- ↑ Ramakrishnan, B.; Sekharudu, C.; Pan, B.; Sundaralingam, M. (2003). "Near-atomic resolution crystal structure of an A-DNA decamer d(CCCGATCGGG): cobalt hexammine interaction with A-DNA". Acta Crystallogr. D59: 67–72. PMID 12499541.
- ↑ Rudisser, S.; Tinoco, I., Jr. (2000). "Solution structure of Cobalt(III)hexammine complexed to the GAAA tetraloop, and metal-ion binding to G.A mismatches.". J. Mol. Biol. 295: 1211–1232. doi:10.1006/jmbi.1999.3421. PMID 10653698.
- ↑ McPherson,, Alexander (2002). Introduction to Macromolecular Crystallography. John Wiley & Sons. ISBN 0-471-25122-4.
