Fast parallel proteolysis
Fast parallel proteolysis (FASTpp) is a method to determine the thermostability of proteins that does not need previous purification and labelling.[1]
History and background
Proteolysis is widely used in biochemistry and cell biology to probe protein structure.[2][3] In "limited trypsin proteolysis", low amounts of protease digest both folded and unfolded protein but at largely different rates: unstructured proteins are cut more rapidly, while structured proteins are cut at a slower rate (sometimes by orders of magnitude). Recently, several other assays of protein stability based on proteolysis have been proposed, exploiting other proteases with high specificity for cleaving unfolded proteins. These include Pulse Proteolysis,[4] Proteolytic Scanning Calorimetry [5] and FASTpp.
How it works
FASTpp measures the quantity of protein that resists digestion under various conditions. To this end, a thermostable protease is used, which cleaves specifically at exposed hydrophobic residues. The FASTpp assay combines the thermal unfolding, specificity of a thermostable protease for the unfolded fraction with the separation power of SDS-PAGE.[6] Due to this combination, FASTpp can detect changes in the fraction folded over a large physico-chemical range of conditions including temperatures up to 85 °C, pH 6-9, presence or absence of the whole cytosolic proteome. Applications range from biotechnology to study of point mutations and ligand binding assays.
Applications
FASTpp has been used to probe:[1]
- Lysate effect on protein stability
- Coupled folding and binding [7]
- Ligand effects on fraction folded & stability [8]
- Effects of mutations on fraction folded & stability (e.g. point mutation/missense mutationss[8][9])
- Kinetic protein stability [10]
Technology
Principles
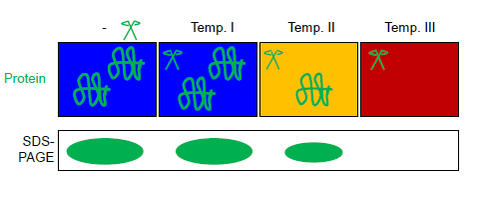
A protein mixture is aliquoted into several tubes, which are exposed in parallel to different temperatures and a thermostable protease (see figure). Automated temperature control is achieved in a thermal gradient cycler (commonly used for PCRs). Reaction products can be separated by SDS-PAGE or western blot.[6] The protease thermolysin can be fully inactivated by EDTA. This feature of thermolysin makes FASTpp compatible with subsequent trypsin digestion e.g. for mass spectrometry.[11][12]
References
- 1 2 Minde, D. P.; Maurice, M. M.; Rüdiger, S. G. D. (2012). Uversky, Vladimir N, ed. "Determining Biophysical Protein Stability in Lysates by a Fast Proteolysis Assay, FASTpp". PLoS ONE. 7 (10): e46147. doi:10.1371/journal.pone.0046147. PMC 3463568
 . PMID 23056252.
. PMID 23056252. - ↑ Johnson, D. E.; Xue, B.; Sickmeier, M. D.; Meng, J.; Cortese, M. S.; Oldfield, C. J.; Le Gall, T.; Dunker, A. K.; Uversky, V. N. (2012). "High-throughput characterization of intrinsic disorder in proteins from the Protein Structure Initiative". Journal of Structural Biology. 180 (1): 201–215. doi:10.1016/j.jsb.2012.05.013. PMC 3578346
 . PMID 22651963.
. PMID 22651963. - ↑ Hoelen, H.; Kleizen, B.; Schmidt, A.; Richardson, J.; Charitou, P.; Thomas, P. J.; Braakman, I. (2010). Uversky, Vladimir N, ed. "The Primary Folding Defect and Rescue of ΔF508 CFTR Emerge during Translation of the Mutant Domain". PLoS ONE. 5 (11): e15458. doi:10.1371/journal.pone.0015458. PMC 2994901
 . PMID 21152102.
. PMID 21152102. - ↑ Park, C.; Marqusee, S. (2005). "Pulse proteolysis: A simple method for quantitative determination of protein stability and ligand binding". Nature Methods. 2 (3): 207–212. doi:10.1038/nmeth740. PMID 15782190.
- ↑ Tur-Arlandis, G.; Rodriguez-Larrea, D.; Ibarra-Molero, B.; Sanchez-Ruiz, J. M. (2010). "Proteolytic Scanning Calorimetry: A Novel Methodology that Probes the Fundamental Features of Protein Kinetic Stability". Biophysical Journal. 98 (6): L12–L14. doi:10.1016/j.bpj.2009.11.028. PMC 2849053
 . PMID 20303845.
. PMID 20303845. - 1 2 Laemmli, U. K. (1970). "Cleavage of structural proteins during the assembly of the head of bacteriophage T4". Nature. 227 (5259): 680–685. doi:10.1038/227680a0. PMID 5432063.
- ↑ Demarest, S. J.; Martinez-Yamout, M.; Chung, J.; Chen, H.; Xu, W.; Dyson, H. J.; Evans, R. M.; Wright, P. E. (2002). "Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators". Nature. 415 (6871): 549–553. doi:10.1038/415549a. PMID 11823864.
- 1 2 Robaszkiewicz, K.; Ostrowska, Z.; Cyranka-Czaja, A.; Moraczewska, J. (2015). "Impaired tropomyosin–troponin interactions reduce activation of the actin thin filament". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1854: 381–390. doi:10.1016/j.bbapap.2015.01.004.
- ↑ Minde, D. P.; Anvarian, Z.; Rüdiger, S. G.; Maurice, M. M. (2011). "Messing up disorder: How do missense mutations in the tumor suppressor protein APC lead to cancer?". Molecular Cancer. 10: 101. doi:10.1186/1476-4598-10-101. PMC 3170638
 . PMID 21859464.
. PMID 21859464. - ↑ Tur-Arlandis, G.; Rodriguez-Larrea, D.; Ibarra-Molero, B.; Sanchez-Ruiz, J. M. (2010). "Proteolytic Scanning Calorimetry: A Novel Methodology that Probes the Fundamental Features of Protein Kinetic Stability". Biophysical Journal. 98 (6): L12–L14. doi:10.1016/j.bpj.2009.11.028. PMC 2849053
 . PMID 20303845.
. PMID 20303845. - ↑ Chang, Y; Schlebach, JP; Verheul, RA; Park, C (2012). "Simplified proteomics approach to discover protein-ligand interactions". Protein science : a publication of the Protein Society. 21 (9): 1280–7. doi:10.1002/pro.2112. PMID 22733688.
- ↑ Park, C; Marqusee, S (2005). "Pulse proteolysis: A simple method for quantitative determination of protein stability and ligand binding". Nature Methods. 2 (3): 207–12. doi:10.1038/nmeth740. PMID 15782190.
External links
- Susan Marqusee lab: http://zebra.berkeley.edu/ (developed Pulse Proteolysis).
- Chiwook Park lab: http://www.mcmp.purdue.edu/faculty/?uid=chiwook (novel Pulse Proteolysis applications in drug discovery and systems biology).
- Jose Manuel Sanchez Ruiz lab: http://prodestech.cib.csic.es/members/group-2 (Proteolytic scanning calorimetry).
- James Bardwell lab: http://labs.mcdb.lsa.umich.edu/labs/bardwell/ (genetically encoded in vivo proteolysis assays for e.g. chaperone discovery).
