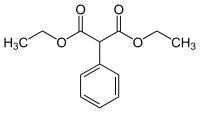
Diethyl phenylmalonate
 | |
| Names | |
|---|---|
| IUPAC name
Diethyl phenylmalonate | |
| Other names
Diethyl phenylpropanedioate; Propanedioic acid 2-phenyl- diethyl ester; Diethyl-phenylmalonat | |
| Identifiers | |
| 83-13-6 | |
| 3D model (Jmol) | Interactive image |
| 614465 | |
| ChemSpider | 59885 |
| ECHA InfoCard | 100.001.324 |
| EC Number | 201-456-5 |
| PubChem | 66514 |
| |
| |
| Properties | |
| C13H16O4 | |
| Molar mass | 236.27 g·mol−1 |
| Density | 1.096 g/cm3 |
| Melting point | 16.5 °C (61.7 °F; 289.6 K) |
| Boiling point | 170–172 °C (338–342 °F; 443–445 K) (14 mmHg) |
| Refractive index (nD) |
n20/D 1.491 |
| Hazards | |
| Safety data sheet | MSDS |
| Flash point | 120 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diethyl phenylmalonate is an aromatic malonic ester used in the synthesis of moderate to long lasting barbiturates such as phenobarbital.[1]
Chemical synthesis
Unlike other malonic esters that are derived via malonic ester synthesis, diethyl phenylmalonate is typically indirectly derived via a Claisen condensation with diethyl oxalate and ethyl phenylacetate followed by decarbonylation.[2] This indirect method is often used because aryl halides are relatively weaker nucleophiles than aliphatic halocarbons and thus poorly alkylate diethyl malonate.[3] Methods using Caesium carbonate and copper(I) iodide have been developed to overcome this difficulty however.[4]
References
- ↑ Wollweber, Hartmund (2000). "Hypnotics". Ullmann's Encyclopedia of Industrial Chemistry: 11. doi:10.1002/14356007.a13_533.
- ↑ Meyer, G. M.; Levene, P. A. (1936). "Diethyl phenylmalonate". Organic Syntheses. 16: 33. doi:10.15227/orgsyn.016.0033.
- ↑ Furniss, Brian; Hannaford, Antony; Smith, Peter; Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed. London: Longman Science & Technical. pp. 1174–1179. ISBN 9780582462366.
- ↑ Hennessy, Edward J.; Buchwald, Stephen L. (2002). "A General and Mild Copper-Catalyzed Arylation of Diethyl Malonate". Organic Letters. 4 (2): 269–272. doi:10.1021/ol017038g.
This article is issued from Wikipedia - version of the 5/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.