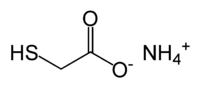
Ammonium thioglycolate
 | |
| Names | |
|---|---|
| Other names
Perm salt | |
| Identifiers | |
| 5421-46-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 20239 |
| ECHA InfoCard | 100.024.128 |
| |
| |
| Properties | |
| C2H7NO2S | |
| Molar mass | 109.15 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ammonium thioglycolate, also known as perm salt, is the salt of thioglycolic acid and ammonia. It has the formula HSCH2CO2NH4 and has use in perming hair.[1]
Chemistry
Being the salt of a weak acid and weak base, ammonium thioglycolic acid exists in solution as an equilibrium mixture of the salt itself as well as thioglycolic acid and ammonia:
Thioglycolate, in turn, is able to cleave disulfide bonds, capping one side with a hydrogen and forming a new disulfide with the other side:
- RSH + R'SSR' ⇌ R'SH + RSSR'
The chemistry of perms
A solution containing ammonium thioglycolate contains a lot of free ammonia, which swells hair, rendering it permeable. The thioglycolic acid in the perm solution reduces the disulfide cystine bonds in the cortex of the hair.[2] In a sense, the thioglycolate removes crosslinks. After washing, the hair is treated with a mild solution of hydrogen peroxide, which oxidizes the cysteines back to cystine. These new chemical bonds impart the structural rigidity necessary for a successful perm. The rigidification process is akin to the vulcanization of rubber, where commonly polysulfide linkages are used to crosslink the polymer chains. However, not as many disulfide bonds are reformed as there were before the permanent. As a result, the hair is weaker than before the permanent was applied and repeated applications over the same spot may eventually cause strand breakage.
Since polar molecules are less volatile than nonpolar ones, the glycolate substituent makes the thiol non-volatile and hence less odorous. An added advantage is that the glycolate confers some solubility in water. One could almost certainly use HSCH3 and ammonia to give a perm, but there would be serious olfactory consequences.
References
- ↑ United States' National Library of Medicine: Ammonium Thioglycolate
- ↑ Robbins, Clarence R. (2000), Chemical and Physical Behavior of Human Hair, 4th ed, pp. 106–108
