Trichuris trichiura
| Whipworm(s) | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Enoplea |
| Order: | Trichocephalida |
| Family: | Trichuridae |
| Genus: | Trichuris |
| Species: | T. trichiura |
| Binomial name | |
| Trichuris trichiura (Linnaeus, 1771) | |
The human whipworm (Trichuris trichiura or Trichocephalus trichiuris) is a round worm (a type of helminth) that causes trichuriasis (a type of helminthiasis which is one of the neglected tropical diseases) when it infects a human large intestine. It is commonly known as the whipworm which refers to the shape of the worm; it looks like a whip with wider "handles" at the posterior end.
Life cycle
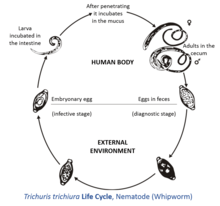
The female T. trichiura produces 2,000–10,000 single-celled eggs per day.[1] Eggs are deposited from human feces to soil where, after two to three weeks, they become embryonated and enter the “infective” stage. These embryonated infective eggs are ingested and hatch in the human small intestine exploiting the intestinal microflora as hatching stimulus.[2] This is the location of growth and molting. The infective larvae penetrate the villi and continue to develop in the small intestine. The young worms move to the caecum and penetrate the mucosa and there they complete development to adult worms in the large intestine. The life cycle from time of ingestion of eggs to development of mature worms takes approximately three months. During this time, there may be limited signs of infection in stool samples due to lack of egg production and shedding. The female T. trichiura begin to lay eggs after three months of maturity. Worms can live up to five years, during which time females can lay up to 20,000 eggs per day.
Recent studies using genome-wide scan revealed two quantitative trait loci on chromosome 9 and chromosome 18 may be responsible for genetic predisposition or susceptibility to infection of T. trichiura by some individuals.
Morphology
Trichuris trichiura has a narrow anterior esophageal end and shorter and thicker posterior anus. These pinkish-white worms are threaded through the mucosa. They attach to the host through their slender anterior end and feed on tissue secretions instead of blood. Females are larger than males; approximately 35–50 mm long compared to 30–45 mm.[3] The females have a bluntly round posterior end compared to their male counterparts with a coiled posterior end. Their characteristic eggs are barrel-shaped and brown, and have bipolar protuberances..
Infection
Infection occurs through ingestion of eggs and is more common in warmer areas. Whipworms eggs are passed in the feces of infected persons, and if an infected person defecates outside or if untreated human feces as used as fertilizer, eggs are deposited on soil where they can mature into an infective stage. Ingestion of these eggs "can happen when hands or fingers that have contaminated dirt on them are put in the mouth or by consuming vegetables or fruits that have not been carefully cooked, washed or peeled."[4] The eggs hatch in the small intestine, and then move into the wall of the small intestine and develop. On reaching adulthood, the thinner end (the front of the worm) burrows into the large intestine and the thicker end hangs into the lumen and mates with nearby worms. The females can grow to 50 mm (2.0 in) long. Neither the male nor the female has much of a visible tail past the anus.[1]
Whipworm commonly infects patients also infected with Giardia, Entamoeba histolytica, Ascaris lumbricoides, and hookworms.
Epidemiology
There is a worldwide distribution of Trichuris trichiura, with an estimated 1 billion human infections.[5][6][7] However, it is chiefly tropical, especially in Asia and, to a lesser degree, in Africa and South America. Within the United States, infection is rare overall but may be common in the rural Southeast, where 2.2 million people are thought to be infected. Poor hygiene is associated with trichuriasis as well as the consumption of shaded moist soil, or food that may have been fecally contaminated. Children are especially vulnerable to infection due to their high exposure risk. Eggs are infective about 2–3 weeks after they are deposited in the soil under proper conditions of warmth and moisture, hence its tropical distribution.
Other animals
Whipworms develop when a dog swallows whipworm eggs, passed from an infected dog. Symptoms may include diarrhea, anemia, and dehydration. The dog whipworm (Trichuris vulpis) is commonly found in the U.S. It is hard to detect at times, because the numbers of eggs shed are low, and they are shed in waves. Centrifugation is the preferred method. There are several preventives available by prescription from a veterinarian to prevent dogs from getting whipworm.
The cat whipworm is a rare parasite. In Europe, it is represented mostly by Trichuris campanula, and in North America it is Trichuris serrata more often.[8][9] Whipworm eggs found in cats in North America must be differentiated from lungworms, and from mouse whipworm eggs just passing through.
Treatment of inflammatory disorders
The hygiene hypothesis suggests that various immunological disorders that have been observed in humans only within the last 100 years, such as Crohn's disease, or that have become more common during that period as hygienic practices have become more widespread, may result from a lack of exposure to parasitic worms (also called helminths) during childhood. The use of Trichuris suis ova (TSO, or pig whipworm eggs) by Weinstock, et al., as a therapy for treating Crohn's disease[10][11][12] and to a lesser extent ulcerative colitis[13] are two examples that support this hypothesis. There is also anecdotal evidence that treatment of inflammatory bowel disease (IBD) with TSO decreases the incidence of asthma,[14] allergy,[15] and other inflammatory disorders. Some scientific evidence suggests that the course of multiple sclerosis may be very favorably altered by helminth infection;[16] TSO is being studied as a treatment for this disease.[17][18]
References
- 1 2 Cross, John H. (1996). "Enteric Nematodes of Humans". In Baron, Samuel. Medical Microbiology (4th ed.). Galveston: University of Texas Medical Branch at Galveston. ISBN 0-9631172-1-1.
- ↑ Hayes, K. S.; Bancroft, A. J.; Goldrick, M.; Portsmouth, C.; Roberts, I. S.; Grencis, R. K. (2010). "Exploitation of the Intestinal Microflora by the Parasitic Nematode Trichuris muris". Science. 328 (5984): 1391–4. doi:10.1126/science.1187703. PMC 3428897
 . PMID 20538949.
. PMID 20538949. - ↑ "Trichuris trichiura definition - Medical Dictionary definitions of popular medical terms easily defined on MedTerms". Medterms.com. 2000-04-15. Retrieved 2009-05-19.
- ↑ http://www.cdc.gov/parasites/whipworm/[]
- ↑ Crompton, DW (1999). "How much human helminthiasis is there in the world?". The Journal of Parasitology. 85 (3): 397–403. doi:10.2307/3285768. PMID 10386428.
- ↑ de Silva, Nilanthi R; Brooker, Simon; Hotez, Peter J; Montresor, Antonio; Engels, Dirk; Savioli, Lorenzo (2003). "Soil-transmitted helminth infections: updating the global picture". Trends in Parasitology. 19 (12): 547–51. doi:10.1016/j.pt.2003.10.002. PMID 14642761.
- ↑ "Trichuris trichiura". WrongDiagnosis.com. 2009-05-06. Retrieved 2009-05-19.
- ↑ "Whipworms". VeterinaryPartner.com. 24 September 2007. Retrieved 2009-05-19.
- ↑ Hendrix CM, Blagburn BL, Lindsay DS (1987). "Whipworms and intestinal threadworms". Vet. Clin. North Am. Small Anim. Pract. 17 (6): 1355–75. PMID 3328393.
- ↑ Hunter MM, McKay DM (2004). "Review article: helminths as therapeutic agents for inflammatory bowel disease". Aliment. Pharmacol. Ther. 19 (2): 167–77. doi:10.1111/j.0269-2813.2004.01803.x. PMID 14723608.
- ↑ Summers RW, Elliott DE, Urban JF, Thompson R, Weinstock JV (2005). "Trichuris suis therapy in Crohn's disease". Gut. 54 (1): 87–90. doi:10.1136/gut.2004.041749. PMC 1774382
 . PMID 15591509.
. PMID 15591509. - ↑ Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R, Weinstock JV (2003). "Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease". Am. J. Gastroenterol. 98 (9): 2034–41. doi:10.1111/j.1572-0241.2003.07660.x. PMID 14499784.
- ↑ Buning, J; et al. (March 2008). "Helminths as governors of inflammatory bowel disease". Gut. 57 (8): 1182–1183. doi:10.1136/gut.2008.152355. PMID 18628388. Retrieved 2010-12-10.
in our patient Treg [regulatory T cells] activated by helminthosis [T. suis infestation] were most likely the key element protecting a host with latent ulcerative colitis against development of a severe protcocolitis. (1183)
- ↑ "Helminthic Therapy: How to put your Asthma, Colitis, IBD, Crohn's or Multiple Sclerosis into remission with hookworm". Asthmahookworm.com. Retrieved 2009-05-19.
- ↑ "Allergies: Trichuris suis Ova (TSO) Therapy to Treat Food Allergies". Allergizer.com. Retrieved 2009-05-19.
- ↑ Correale J, Farez M (2007). "Association between parasite infection and immune responses in multiple sclerosis". Annals of Neurology. 61 (2): 97–108. doi:10.1002/ana.21067. PMID 17230481.
- ↑ "Asphelia Announces Initiation of an Independent TSO Trial for Multiple Sclerosis". redOrbit. 2008-04-07. Retrieved 2009-05-19.
- ↑ Klaver, Elsenoor J.; Kuijk, Loes M.; Laan, Lisa C.; Kringel, Helene; van Vliet, Sandra J.; Bouma, Gerd; Cummings, Richard D.; Kraal, Georg; van Die, Irma (2013). "Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated". International Journal for Parasitology. 43 (3-4): 191–200. doi:10.1016/j.ijpara.2012.10.021. PMID 23220043.
External links
| Wikimedia Commons has media related to Trichuris trichiura. |
- Trichuris Trichiura at eMedicine
- Man finds extreme healing eating parasitic worms, By Elizabeth Cohen, CNN Senior Medical CorrespondentDecember 9, 2010
- Potential Disease Treatment: Swallow Some Worms
- Globe and Mail: Sometimes having worms is good
- BBC article mentions the Iceman had Whipworm
- Video of Live Trichuris trichiura "Whip Worm"