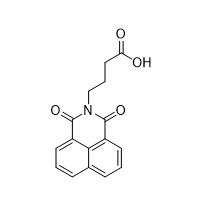
Virstatin
 | |
| Identifiers | |
|---|---|
| |
| PubChem (CID) | 145949 |
| ChemSpider | 128749 |
| ChEMBL | CHEMBL403272 |
| Chemical and physical data | |
| Formula | C16H13NO4 |
| Molar mass | 283.28 g/mol |
| 3D model (Jmol) | Interactive image |
| Density | 1.40 g/cm3 |
| Melting point | 86–88 °C (187–190 °F) |
| Boiling point | 536.52 °C (997.74 °F) |
| |
| |
Virstatin is a drug aimed at combating cholera.[1] Rather than target the disease-causing bacterium (Vibrio cholerae) itself, the drug targets the genes involved in virulence. Virstatin prevents the bacteria from producing the toxin that causes diarrhea, as well as inhibits the bacterium from colonizing the intestine.[2] The drug is unique as it does not target DNA, RNA, or protein synthesis.[3] The drug is specific to Vibrio cholerae. The drug's efficacy has been demonstrated in mice models. It has been shown to both prevent cholera when used prophylactically, as well as shorten the length of the disease once the organism is afflicted. By halting the uncontrolled spread of the disease, the body can more easily rid the bacterium.
Discovery
In 2005, researchers at Harvard University discovered that virstatin could be a viable drug candidate for treatment of cholera. The research was led by John Mekalanos, professor and chair of the Department of Microbiology and Molecular Genetics, and Deborah Hung, an instructor in medicine.[3] The researchers' initial goal was identify molecules that targeted virulence factors in an effort to find new drugs that can evade antimicrobial resistance.[1] High-throughput screening was performed on a library of approximately 50,000 molecules in order to identify virulence factor inhibitors. Fifteen compounds were then selected due to their effect on virulence factors with only minor effects on bacteria survival. From there, virstatin was chosen and then synthesized in order for characterization of its activity.
Mechanism of Action
Virstatin acts as an inhibitor of the transcriptional activator ToxT. When ToxT is activated, the ctx and tcp genes are expressed.[4] The ctx gene codes for the cholera toxin (CT) while the tcp gene codes for the toxin coregulated pilus (TCP). Cholera toxin results in diarrhea in affected individuals. Toxin coregulated pilus allows for intestinal colonization by the bacterium.

Virstatin acts to inhibit ToxT dimerization.[5] The proposed model hypothesizes that virstatin prevents the N-terminal of ToxT from interacting with the N-terminal of another ToxT in order to form a dimer. The ctx and tcp promoters are dependent on the dimerization of ToxT. Once ToxT becomes a dimer, it is able to bind the promoter and allows for initiation of transcription of the genes.
Inhibition of ToxT dimerization prevents the transcription of ctx and tcp. With no transcription, there is no translation and thus no expression of the deleterious cholera toxin and toxin coregulated pilus.
In addition to virstatin, fatty acids have also been shown to regulate the virulence genes in Vibrio cholerae.[6] More specifically, the unsaturated fatty acid cis-palmitoleate reduces CT and TCP expression. This discovery was made when analyzing the crystal structure of ToxT. This research has implications in further understanding the interactions between virstatin and ToxT. Another unsaturated fatty acid, linoleic acid, affects ToxT binding to promoters. Linoleic acid was shown to affect ToxT binding promoters have either one or two ToxT binding sites, suggesting unsaturated fatty acids affect monomeric ToxT binding to DNA.[7] Unlike linoleic acid, virstatin affects DNA binding at ToxT promoters with two binding sites.

Laboratory synthesis
The compound was synthesized in gram amounts in order to quantify its activity after initial discovery in 2005. The researchers synthesized virstatin by reacting 1,8-napthalic anhydride with 4-amino-n-butyric acid.[1]

A simple two-step synthesis of virstatin was also demonstrated for implementation in a teaching laboratory.[8] The first step involves the reaction of 1,8-naphthalimide with ethyl-4-bromobutyrate in the presence of potassium carbonate. The exocyclic ester was then hydrolyzed with potassium hydroxide followed by an aqueous workup.
Resistance to the drug
The L113P mutant of ToxT is resistant to virstatin's effects.[1] Studies have shown that this ToxT mutant successfully dimerizes in the presence of virstatin, as the mutation most likely results in more open conformation which allows for interactions to occur between subunits.[5] Site-directed mutagenesis studies discovered that both an L114A mutation and an N106F mutation in ToxT allow dimerization in the presence of virstatin.[9] The region that contains these two amino acids was determined to an N-terminal unstructured region that is important for interactions with both positive and negative effectors.
Resistance was also seen in different serotypes of the bacteria. The O1 and O139 serogroups of Vibrio cholerae are the two most common, however other strains do result in some endemics seen worldwide. The initial research on virstatin was done using the O1 and O139 serogroups.[1] Various other serotypes were tested for virstatin resistance and these strains were further characterized. Five of those strains were shown to have only 62-64% N-terminal ToxT homology as compared to the ToxT proteins in the O1 and O139 serogroups.[10] These results are consistent with previous data on the drug's interactions with ToxT, as the virstatin binds in a sequence-dependent manner to inhibit ToxT dimerization.
Interactions with other proteins
Human serum albumin functions as a transporter of many molecules, including drugs. FRET and ITC studies indicated that virstatin binds Site I (subdomain IIA) in human serum albumin.[11] also known as the warfarin binding site.
Virstatin also binds to accessory cholera enterotoxin (Ace), another toxin derived from Vibrio cholerae, thought to be involved in fluid secretion and reduces its propensity to form an alpha helix.[12][13]
Effect on other bacteria
Virstatin has also been shown to inhibit biofilm formation of Actinetobacter baumannii. It does this by decreasing pili expression, which are important in the development of biofilms.[14]
Future research
In addition to virstatin, 14 other compounds were identified as potential drugs by high-throughput screening (3). These compounds have the potential to be successful in combating cholera as well. Further evaluation is also needed before virstatin can be considered for use in humans.
References
- 1 2 3 4 5 Hung, D. T. (2005). "Small-Molecule Inhibitor of Vibrio cholerae Virulence and Intestinal Colonization". Science. 310 (5748): 670–674. doi:10.1126/science.1116739. ISSN 0036-8075. PMID 16223984.
- ↑ "New Antibiotic Could Fight Cholera". Consumer HealthDay. Retrieved 2016-04-19.
- 1 2 "New cholera drug shows wider potential". UPI. Retrieved 2016-04-19.
- ↑ Stonehouse, E. A.; Hulbert, R. R.; Nye, M. B.; Skorupski, K.; Taylor, R. K. (2011). "H-NS binding and repression of the ctx promoter in Vibrio cholerae". J. Bacteriol. 193 (4): 979–988. doi:10.1128/JB.01343-09. PMC 3028689
 . PMID 21169492.
. PMID 21169492. - 1 2 Shakhnovich, E. A.; Hung, D. T.; Pierson, E.; Lee, K.; Mekalanos, J. J. (2007). "Virstatin inhibits dimerization of the transcriptional activator ToxT". Proc. Natl. Acad. Sci. U.S.A. 104 (7): 2372–2377. doi:10.1073/pnas.0611643104. PMID 17283330.
- ↑ Lowden, M. J.; Skorupski, K.; Pellegrini, M.; Chiorazzo, M. G.; Taylor, R. K.; Kull, F. J (2010). "Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes". Proc. Natl. Acad. Sci. U.S.A. 107 (7): 2860–2865. doi:10.1073/pnas.0915021107. PMID 20133655.
- ↑ Plecha, S. C.; Withey, J. H (2015). "Mechanism for Inhibition of Vibrio cholerae ToxT Activity by the Unsaturated Fatty Acid Components of Bile". J. Bacteriol. 197 (10): 1716–1725. doi:10.1128/JB.02409-14. PMID 25733618.
- ↑ McDonald, C. E. (2009). "A Two-Step Synthesis of Virstatin, A Virulence Inhibitor of Vibrio cholerae". J. Chem. Educ. 86 (4): 482. doi:10.1021/ed086p482.
- ↑ Thomson, J. J.; Plecha, S. C.; Withey, J. H. (2015). "A Small Unstructured Region in Vibrio cholerae ToxT Mediates the Response to Positive and Negative Effectors and ToxT Proteolysis". J. Bacteriol. 197 (3): 654–668. doi:10.1128/JB.02068-14. PMC 4285994
 . PMID 25422303.
. PMID 25422303. - ↑ Shakhnovich, E. A.; Sturtevant, D.; Mekalanos, J. (2007). "Molecular mechanisms of virstatin resistance by non-O1/non-O139 strains of Vibrio cholerae". J. Mol. Microbiol. 66 (6): 1331–1341. doi:10.1111/j.1365-2958.2007.05984.x.
- ↑ Chatterjee, T.; Pal, A.; Dey, S.; Chatterjee, B. K.; Chakrabarti, P. (2012). "Interaction of Virstatin with Human Serum Albumin: Spectroscopic Analysis and Molecular Modeling". PLoS ONE. 7 (5): e37468. doi:10.1371/journal.pone.0037468.
- ↑ Trucksis, M.; Galen, J. E.; Michalski, J.; Fasano, A.; Kaper, J. B. (1993). "Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette". Proc. Natl. Acad. Sci. U.S.A. 90 (11): 5267–5271. doi:10.1073/pnas.90.11.5267. PMC 46697
 . PMID 8389476.
. PMID 8389476. - ↑ Chatterjee, T.; Mukherjee, D.; Dey, S.; Pal, A.; Hoque, K. M.; Chakrabarti (2011). "Accessory Cholera Enterotoxin, Ace, from Vibrio cholerae : Structure, Unfolding, and Virstatin Binding". Biochemistry. 50 (14): 2962–2972. doi:10.1021/bi101673x. PMID 21366345.
- ↑ Nait Chabane, Y.; Mlouka, M. Ben; Alexandre, S.; Nicol, M.; Marti, S.; Pestel-Caron, M.; Vila, J.; Jouenne, T.; Dé, E. (2014). "Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii". BMC Microbiol. 14 (1): 62. doi:10.1186/1471-2180-14-62.