Uroporphyrinogen III
 | |
| Identifiers | |
|---|---|
| 1976-85-8 | |
| MeSH | Uroporphyrinogen+III |
| PubChem | 1179 |
| Properties | |
| C40H44N4O16 | |
| Molar mass | 836.795 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
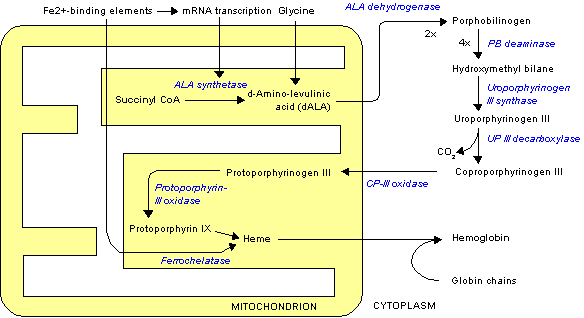
Uroporphyrinogen III is the first cyclic metabolic intermediate in the biosynthesis of heme. The linear precursor, hydroxymethylbilane is generated from four porphobilinogen (PBG) molecules by the third enzyme in the synthesis, hydroxymethylbilane synthase (or porphobilinogen deaminase). When the linear chain of four PBGs (hydroxymethylbilane) is released from hydroxymethylbilane synthase, the next enzyme in the haem biosynthesis, uroporphyrinogen-III synthase, converts hydroxymethylbilane into the cyclic uroporphyrinogen III. This product is subsequently converted into coproporphyrinogen III by the enzyme uroporphyrinogen III decarboxylase. However, if there is no uroporphyrinogen-III synthase present, the linear tetrapyrrole will be spontaneous cyclised into Uroporphyrinogen I, which is then converted into coproporphyrinogen I, also by uroporphyrinogen III decarboxylase
The difference between the two forms, is the arrangements of the four propionic acid ("P" groups) and the four acetic acid groups ("A" groups), where non-enzyamtically conversion into uroporphyrinogen I results in an AP-AP-AP-AP symmetry, whereas the enzymatically conversion into uroporphyrinogen III lead to a reversed AP-group and hence an AP-AP-AP-PA arrangement.
See also