Urea-formaldehyde
Urea-formaldehyde, also known as urea-methanal, so named for its common synthesis pathway and overall structure,[1] is a non-transparent thermosetting resin or plastic. It is produced from urea and formaldehyde. These resins are used in adhesives, finishes, particle board, MDF, and molded objects. UF and related amino resins are a class of thermosetting resins of which urea-formaldehyde resins make up 80% produced globally. Examples of amino resins use include in automobile tires to improve the bonding of rubber to tire cord, in paper for improving tear strength, in molding electrical devices, jar caps, etc.[2]
Properties
Urea-formaldehyde resin's attributes include high tensile strength, flexural modulus, and a high heat distortion temperature, low water absorption, mould shrinkage, high surface hardness, elongation at break, and volume resistance. Index of Refraction = 1.55[3]
Chemical structure
The chemical structure of UF polymer consists of [(O)CNCH2]n repeat units. In contrast melanine-formaldehyde resins feature NCH2OCH2N repeat units. Depending on the polymerization conditions, some branching can occur. Early stages in the reaction of formaldehyde and urea produce bis(hydroxymethyl)urea .
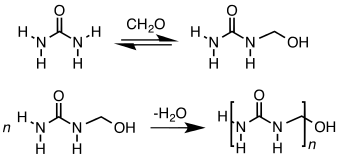
Production
Approximately 1 million metric tons of urea-formaldehyde are produced annually. Over 70% of this production is then put into use by the forest products industry for bonding particleboard (61%), medium density fiberboard (27%), hardwood plywood (5%), and laminating adhesive (7%).
General uses
Urea-formaldehyde is pervasive. Examples include decorative laminates, textiles, paper, foundry sand molds, wrinkle resistant fabrics, cotton blends, rayon, corduroy, etc. It is also used to glue wood together. Urea formaldehyde was commonly used when producing electrical appliances casing (e.g. desk lamps).
Agricultural use
Urea formaldehyde is also used in agriculture as a controlled release source of nitrogen fertilizer. Urea formaldehyde’s rate of decomposition into CO2 and NH
3 is determined by the action of microbes found naturally in most soils. The activity of these microbes, and, therefore, the rate of nitrogen release, is temperature dependent. The optimum temperature for microbe activity is approximately 70-90 °F (approx 20-30 °C).
Foam insulation

Urea-formaldehyde foam insulation (UFFI) dates to the 1930s and made a synthetic insulation with R-values near 5.0 per inch. It is a foam, like shaving cream, that is easily injected or pumped into walls. It is made by using a pump set and hose with a mixing gun to mix the foaming agent, resin and compressed air. The fully expanded foam is pumped into areas in need of insulation. It becomes firm within minutes but cures within a week. UFFI is generally found in homes built before the 1970s, often in basements, crawl spaces, attics, and unfinished attics. Visually it looks like oozing liquid that has been hardened. Over time, it tends to vary in shades of butterscotch but new UFFI is a light yellow color. Early forms of UFFI tended to shrink significantly. Modern UF insulation with updated catalysts and foaming technology have reduced shrinkage to minimal levels (between 2-4%). The foam dries with a dull matte color with no shine. When cured, it often has a dry and crumbly texture.
Safety concerns
Urea-formaldehyde foam insulation (UFFI) was used extensively in the 1970s. Homeowners used UFFI as a wall cavity filler at the time in order to conserve energy. In the 1980s, concerns began to develop about formaldehyde vapor emitted in the curing process, as well as from the breakdown of old foam. Emission rates exceeding 3.0 - 5.0 parts per million (ppm) cause a variety of adverse health effects impacting the eyes, nose, and respiratory system. Consequently, its use was discontinued. The urea-formaldehyde emissions decline over time and significant levels should no longer be present in the homes today.[4] Modern replacement options for UFFI include melamine formaldehyde resin, low-emission UF insulation materials, polycarbonate and polyurethane. The curing temperature is unknown.
UFFI was usually mixed at the location of use while constructing the home’s walls. It was then injected inside the walls, the curing process occurs, and the final product acts as an insulating agent. Because less information was known about the toxic health effects of formaldehyde in the 1970s, extra formaldehyde was often added to the mixture to ensure that the curing process would occur completely. Since the UFFI was not a well-sealed product [open-celled foam], any excess formaldehyde in the insulation would off-gas into the home's living space. The early UFFI materials were also affected by moisture and heat which compounded the offgassing concerns. When temperature rises, residuals of formaldehyde contained in the insulation are released and migrate into indoor air. Remedial actions to take when formaldehyde levels exceed recommended levels include sealing off the any outlets for the vapors; sealing any cracks or openings in interior walls; removing any sources of water or moisture that come in contact with the insulation; applying one or more layers of vapor-barrier paint; increasing the air exchange rate with outside air in buildings that are tightly sealed; or covering walls with Mylar or vinyl paper. Aluminum foil is a useful alternative for barricading vapors. Generally there is not an off-gassing concern with older UFFI insulation, since those materials have already cured. Removal is a costly and tedious option for UFFI, and it requires the installation of replacement insulation.
Health concerns
Health effects occur when urea-formaldehyde based materials and products release formaldehyde into the air. Generally there are no observable health effects from formaldehyde when air concentrations are below 1.0 ppm. The onset of respiratory irritation and other health effects, and even increased cancer risk begins when air concentrations exceed 3.0-5.0 ppm. This triggers watery eyes, nose irritations, wheezing and coughing, fatigue, skin rash, severe allergic reactions, burning sensations in the eyes and throat, nausea, and difficulty in breathing in some humans (usually > 1.0 ppm).[5][6] Occupants of UFFI insulated homes with elevated formaldehyde levels experienced systemic symptoms such as headache, malaise, insomnia, anorexia, and loss of libido. Irritation of the mucous membranes (specifically the eyes, nose, and throat) was a common upper respiratory tract symptom related to formaldehyde exposure. However, when compared to control groups, the frequency of symptoms did not exceed the controls except when it came to wheezing, difficult breathing, and a burning skin sensation. Controlled studies have suggested that tolerance to formaldehyde's odor and irritating effects can occur over a prolonged exposure.
History
It was first synthesized in 1884 by Hölzer, who was working with Bernhard Tollens.[7] In 1919, Hanns John (1891–1942) of Prague, Czechoslovakia obtained the first patent for urea-formaldehyde resin.[8]
See also
- Phenol formaldehyde resin
- MDF-Safety aspects
- Urea-formaldehyde was object matter of judgment via the European Court of Justice (now CJEU) of 5 February 1963, Case 26-62 "Van Gend & Loos v Netherlands Inland Revenue Administration"
References
- ↑ Uses Of Formaldehyde
- ↑ H. Deim, G. Matthias, R. A. Wagner "Amino Resins" in Ullmann's Encyclopedia of Industrial Chemistry,2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a02_115.pub2
- ↑ Brady, George S.; Clauser, Henry R. ; Vaccari A., John (1997). Materials Handbook (14th ed.). New York, NY: McGraw-Hill. ISBN 0-07-007084-9. Cite uses deprecated parameter
|coauthors=(help) - ↑ Environmental "Health Center Formaldehyde FAQ" Check
|url=value (help). - ↑ "Press Release N° 153".
- ↑ "Occupational-toxicological risk related to the exposure to MDF wood dust".
- ↑ See:
- Tollens, B. (1884) "Ueber einige Derivate des Formaldehyds" (On some derivatives of formaldehyde), Berichte der Deutschen Chemischen Gesellschaft, 17 : 653-659. On page 659, Tollens mentions in passing: "… , aus Harnstoff und Formaldehyd hat dagegen Dr. Hölzer ein festes, schwer lösliches Derivat erhalten." (… from urea and formaldehyde, on the other hand, Dr. Hölzer obtained a solid, almost insoluble derivative.)
- B. Tollens (1896) "Ueber den Methylen-Harnstoff" (On methylene-urea), Berichte der deutschen chemischen Gesellschaft, 29 (3) : 2751-2752. Neither Hölzer nor Tollens realized that the urea and formaldehyde were polymerizing.
- Goldschmidt, Carl (1896) "Ueber die Einwirkung von Formaldehyd auf Harnstoff" (On the effect of formaldehyde on urea), Berichte der Deutschen Chemischen Gesellschaft, 29 (3) : 2438-2439.
- Goldschmidt, C. (1897) "Ueber die Einwirkung von Formaldehyd auf Harnstoff," Chemiker-Zeitung, 21 (46) : 460.
- ↑ See:
- H. John "Verfahren zur Herstellung von Kondensationsprodukten aus Formaldehyd und Harnstoff bzw. Thioharnstoff oder anderen Harnstoffderivaten" (Process for the production of condensation products from formaldehyde and urea or thiourea or other urea derivatives), Austrian Patent 78,251, October 9, 1919.
- H. John, "Process for the manufacture of condensation products of formaldehyde and carbamide or carbamide derivatives," Great Britain Patent 151,016, January 16, 1922.
- Hanns John, "Manufacture of aldehyde condensation product capable of technical utilization," U.S. Patent 1,355,834, October 19, 1920.
- Urea formaldehyde (Plastics Historical Society)
- History of urea-formaldehyde: Chapter 1 of: Carl Meyer, Urea-Formaldehyde Resins (Reading, Massachusetts: Addison-Wesley, 1979)
- Urea-Formaldehyde Foam Insulation(Canada Mortgage and Housing Corporation)
- Indoor Air Quality: Formaldehyde(US Environmental Protection Agency)
- Formaldehyde.... its safe use in foundries (UK Health and Safety Executive)
- (United States Environmental Protection Agency)
- (Environmental and Occupational Health Assessment Program|Connecticut Department of Public Health)
- (Consumer Product Safety Commission|Consumer Product Safety Commission)
- (Forest Products Laboratory: USDA Forest Service)
- [Dunky, M., “Urea-formaldehyde (UF) adhesive resins for wood,” International Journal of Adhesion and Adhesives, 1998. (18:2).]
- (Encyclopædia Britannica)
- (PropEx.com)
- (U.S. Dept. of Labor, Occupational Safety and Health Administration (OSHA))