Tricin
 | |
 | |
| Names | |
|---|---|
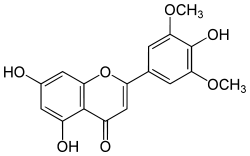
| IUPAC name
5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-4H-chromen-4-one | |
| Other names
Tricetin 3',5'-dimethyl ether 5,7-Dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)chromen-4-one | |
| Identifiers | |
| 520-32-1 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL454320 |
| PubChem | 5281702 |
| |
| Properties | |
| C17H14O7 | |
| Molar mass | 330.29 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tricin is a chemical compound. It is an O-methylated flavone, a type of flavonoid. It can be found in rice bran[1] and sugarcane.[2]
Glycosides
- Tricin 4'-glucoside (Tricin-4'-O-beta-D-glucopyranaoside, CAS number 71855-50-0)
- Tricin 5-glucoside (Tricin 5-O-beta-D-glucopyranoside, CAS number 32769-00-9)
- Tricin 7-O-glucoside (Tricin 7-O-beta-D-glucopyranoside, CAS number 32769-01-0)
Biosynthesis
The biosynthesis of flavones has not yet been elucidated in full; however, most of the mechanistic and enzymatic steps have been discovered and studied. In biosynthesizing tricin, there is first stepwise addition of malonyl CoA via the polyketide pathway and p-coumaroyl Coa via the phenylpropanoid pathway.[3] These additions are mediated by the sequential action of chalcone synthase and chalcone isomerase to yield naringenin chalcone and the flavanone, naringenin, respectively. CYP93G1 of the CYP P450 superfamily in rice then desaturates naringenin into apigenin. After this step, it is proposed that flavonoid 3’5’-hydroxylase (F3’5’H) changes apigenin into tricetin.[4] Upon formation of tricetin, 3’-O-methyltransferase and 5’-O-methyltransferase adds methoxy groups to tricetin to form tricin.
Other compounds formed from tricin
Three flavonolignans derived from tricin have been isolated from oats Avena sativa.[5]
External links
References
- ↑ The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesis in ApcMin mice
- ↑ PMID 27598841
- ↑ Tricin—a potential multifunctional nutraceutical. Zhou J, Ibrahim R. Phytochem., 2010, Rev. 9:413-424
- ↑ CYP93G1 is a flavone synthase II which channels flavanones to the biosynthesis of tricin O-linked conjugates in rice. Lam P, Zhu F, Chan W, Liu H, Lo C. Plant Physiology Preview, 2014.
- ↑ Flavonolignans from Avena sativa. Eva Wenzig, Olaf Kunert, Daneel Ferreira, Martin Schmid, Wolfgang Schühly, Rudolf Bauer and Alois Hiermann, J. Nat. Prod., 2005, 68 (2), pp 289–292