Transition metal alkyne complex
In organometallic chemistry, a transition metal alkyne complex is a coordination compound containing one or more alkyne ligands. Such compounds are intermediates in many catalytic reactions that convert alkynes to other organic products, e.g. hydrogenation and trimerization.[1]
Synthesis
Transition metal alkyne complexes are often formed by the displacement of labile ligands by the alkyne. For example, a variety of cobalt-alkyne complexes may be formed by reaction of the alkyne with dicobalt octacarbonyl.[2]
- Co2(CO)8 + R2C2 → Co2(C2R2)(CO)6 + 2 CO
Many alkyne complexes are produced by reduction of metal halides, e.g. titanocene dichloride and bis(triphenylphosphine)platinum dichloride in the presence of the alkyne:
- Cp2TiCl2 + C2R2 + Mg → Cp2Ti(C2R2) + MgCl2
Structure and Bonding

The coordination of alkynes to transition metals is similar to that of alkenes. The bonding is described by the Dewar-Chatt-Duncanson model. Upon complexation the C-C bond elogates and the alkynyl carbon bends away from 180º. For example in the phenylpropyne complex Pt(PPh3)2(C2)Ph(Me), the C-C distance is 1.277(25) vs 1.20 Å for a typical alkyne. The C-C-C angle distorts 40° from linearity.[3] Because the bending induced by complexation, strained alkynes such as cycloheptyne and cyclooctyne are stabilized by complexation.[4]
In the IR spectra, the C-C vibration of alkynes, which occurs near 2300 cm−1, shifts upon complexation to around 1800 cm−1, indicating a weakening of the C-C bond.
η2-coordination to a single metal center
When bonded side-on to a single metal atom, an alkyne serves as a dihapto usually two-electron donor. For early metal complexes, e.g., Cp2Ti(C2R2), strong π-backbonding into one of the π* antibonding orbitals of the alkyne is indicated. This complex is described as a metallacyclopropene derivative of Ti(IV). For late transition metal complexes, e.g., Pt(PPh3)2(MeC2Ph), the π-backbonding is less prominent, and the complex is assigned oxidation state (0).[5][6]
In some complexes, the alkyne is classified as a four-electron donor. In these cases, both pairs of pi-electrons donate to the metal. This kind of bonding was first implicated in complexes of the type W(CO)(R2C2)3.[7]
η2, η2-coordination bridging two metal centers
Because alkynes have two π bonds, alkynes can form stable complexes in which they bridge two metal centers. The alkyne donates a total of four electrons, with two electrons donated to each of the metals. And example of a complex with this bonding scheme is η2-diphenylacetylene-(hexacarbonyl)dicobalt(0).[6]
Benzyne complexes
Transition metal benzyne complexes represent a special case of alkyne complexes since the free benzynes are not stable in the absence of the metal.[8]
Applications
Metal alkyne species are implicated as intermediates in the hydrogenation of alkynes, e.g. to alkenes. This transformation is conducted on a large scale in refineries, which produce acetylene as an unintended side product in the production of ethylene.
Metal-alkyne complexes are intermediates in the metal-catalyzed trimerization and tetramerizations. Cyclooctatetraene is produced from acetylene via the intermediacy of metal alkyne complexes. Variant of this reaction are exploited for certain niche uses, e.g., synthesis of substituted pyridines.
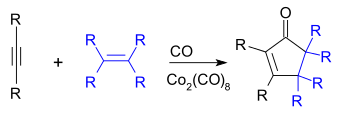
The Pauson-Khand reaction provides a route to cyclopentenones via the intermediacy of cobalt-alkyne complexes.
With the shift away from coal-based (acetylene) to petroleum-based feedstocks (olefins), catalytic reactions with alkynes are not widely practiced industrially. Acrylic acid was once prepared by the hydrocarboxylation of acetylene:[9]
- C2H2 + H2O + CO → H2C=CHCO2H
References
- ↑ Elschenbroich, C. ”Organometallics” 2006 Wiley-VCH: Weinheim. ISBN 3-527-29390-6.
- ↑ Kemmitt, R. D. W.; Russell, D. R.; "Cobalt" in Comprehensive Organometallic Chemistry I; Abel, E.W.; Stone, F.G.A.; Wilkinson, G. eds., 1982, Pergamon Press, Oxford. ISBN 0-08-025269-9
- ↑ William Davies, B.; C. Payne, N., "Studies on metal-acetylene complexes: V. Crystal and molecular structure of bis(triphenylphosphine)(1-phenylpropyne)platinum(0), [P(C6H5)3]2(C6H5CCCH3)Pt0" J. Organomet. Chem. 1975, volume 99, pp. 315. doi:10.1016/S0022-328X(00)88462-4
- ↑ Bennett, Martin A.; Schwemlein, Heinz P. (1989). "Metal Complexes of Small Cycloalkynes and Arynes". Angew. Chem. Int. Ed. Engl. 28 (10): 1296–1320. doi:10.1002/anie.198912961.
- ↑ Hill, A.F. Organotransition Metal Chemistry, 2002, Royal Society of Chemistry, ISBN 0-471-28163-8.
- 1 2 Crabtree, R. H. Comprehensive Organometallic Chemistry V, 2009, John Wiley & Sons, Inc. ISBN 978-0-470-25762-3
- ↑ Joseph L. Templeton "Four-Electron Alkyne Ligands in Molybdenum(II) and Tungsten(II) Complexes" Advances in Organometallic Chemistry 1989, Volume 29, Pages 1–100.doi:10.1016/S0065-3055(08)60352-4
- ↑ William M. Jones, Jerzy Klosin "Transition-Metal Complexes of Arynes, Strained Cyclic Alkynes, and Strained Cyclic Cumulenes" Advances in Organometallic Chemistry 1998, Volume 42, Pages 147–221. doi:10.1016/S0065-3055(08)60543-2
- ↑ W. Bertleff; M. Roeper; X. Sava (2005), "Carbonylation", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a05_217