Tautomer
Tautomers are constitutional isomers of organic compounds that readily interconvert.[1][2][3] This reaction commonly results in the relocation of a proton. Although it is a complicated concept, tautomerism is relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life.
The concept of tautomerizations is called tautomerism. The chemical reaction interconverting the two is called tautomerization.
Examples
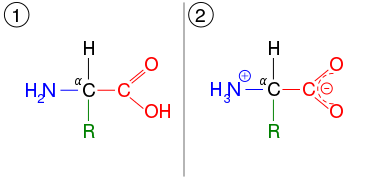
Tautomerization is pervasive in organic chemistry. It is typically associated with polar molecules and ions containing functional groups that are at least weakly acidic. Most commonly tautomers exist in pairs, which means that the proton is located at one of two positions. Common tautomeric pairs include:[4]
- amino acid-ammonium carboxylate, which applies to the building blocks of the proteins.
- ketone - enol, e.g., for acetone (see: keto–enol tautomerism);
- enamine - imine;
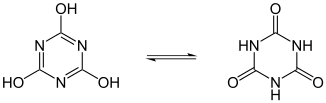
- lactam - lactim, an amide-imidic acid tautomerism in the nucleobases guanine, thymine, and cytosine;
- enamine - enamine, e.g., during pyridoxalphosphate-catalyzed enzymatic reactions; and

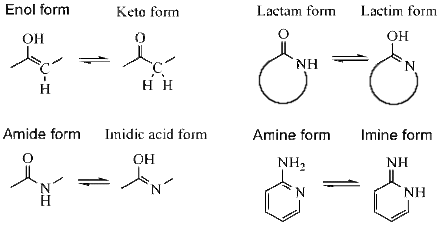
Some more specialized examples are known including nitroso - oxime, ketene - ynol, amide - imidic acid (e.g., the latter is encountered during nitrile hydrolysis reactions);
Prototropy
Prototropy is the most common form of tautomerism and refers to the relocation of a proton.[5] Prototropic tautomerism may be considered as a subset of acid-base behavior. Prototropic tautomers are sets of isomeric protonation states with the same empirical formula and total charge. Tautomerizations are catalyzed by:
- bases, involving a series of steps: deprotonation, formation of a delocalized anion (e.g., an enolate), and protonation at a different position of the anion; and
- acids, involving a series of steps: protonation, formation of a delocalized cation, and deprotonation at a different position adjacent to the cation).
Two specific further subcategories of tautomerizations:
- Annular tautomerism is a type of prototropic tautomerism wherein a proton can occupy two or more positions of a heterocyclic system, for example, 1H- and 3H-imidazole; 1H-, 2H- and 4H- 1,2,4-triazole; 1H- and 2H- isoindole.[6]
- Ring–chain tautomers occur when the movement of the proton is accompanied by a change from an open structure to a ring, such as the open chain and pyran forms of glucose and furan form of fructose.[4]
Valence tautomerism
Valence tautomerism is a type of tautomerism in which single and/or double bonds are rapidly formed and ruptured, without migration of atoms or groups.[7] It is distinct from prototropic tautomerism, and involves processes with rapid reorganisation of bonding electrons. An example of this type of tautomerism can be found in bullvalene. Another example is open and closed forms of certain heterocycles, such as azide - tetrazole or mesoionic münchnone-acylamino ketene. Valence tautomerism requires a change in molecular geometry and should not be confused with canonical resonance structures or mesomers.
See also
References
- ↑ Antonov L (2013). Tautomerism: Methods and Theories (1st ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-33294-6.
- ↑ Smith MB, March J (2001). Advanced Organic Chemistry (5th ed.). New York: Wiley Interscience. pp. 1218–1223. ISBN 0-471-58589-0.
- ↑ Katritzky AR, Elguero J, et al. (1976). The Tautomerism of heterocycles. New York: Academic Press. ISBN 0-12-020651-X.
- 1 2 Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 0-471-72091-7
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Tautomerism".
- ↑ Roman M. Balabin (2009). "Tautomeric equilibrium and hydrogen shifts in tetrazole and triazoles: Focal-point analysis and ab initio limit". J. Chem. Phys. 131 (15): 154307. Bibcode:2009JChPh.131o4307B. doi:10.1063/1.3249968.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Valence tautomerization".