Steroid atrophy
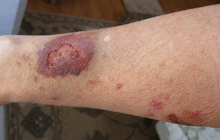
Steroid atrophy, or corticosteroid-induced dermal atrophy, is a side effect of prolonged application of topical corticosteroids. The potential for the condition exists whenever topical corticosteroids are used, even with low potency preparations. Skin atrophy, along with other undesirable side effects such as telangectasia and striae, can appear within 2 to 3 weeks of starting daily application of Class I and II topical corticosteroids, the greatest potential occurring when the application is occluded or when the preparation is applied to fragile skin.[1] Risk depends on the strength of the steroid, the length of application, the site treated, and the nature of the skin problem.[2]
However, it is important to note that recent medical studies have suggested that the fear of "skin thinning" is in many cases unfounded and can lead to poor compliance and treatment failure especially in children as a result of unnecessary fear (see steroid phobia below).
Cause
Within two weeks of starting Topical Steroid treatment, and probably within a few days, microscopic degenerative changes may be seen in the epidermis with a reduction of cell size and the number of cell layers. These effects may be rapidly reversible but with chronic administration, dermal changes become apparent. There is inhibition of the mitotic activity of fibroblasts resulting in reduction of collagen and glycosaminoglycan synthesis but probably the earliest evidence of dermal atrophy is a reduction in the diameter of the fibrils and then the collagen bundles become atrophic and separated. The latter effects have been reported to be caused by the inhibition of collagenase by steroids. Elastin fibres in the upper layers of the dermis become thin and fragmented whereas those deeper down compact into a dense network. As a result of atrophic changes such as striae, telangiectasias, purpura and ecchymosis develop. Long-term use of steroids causes irreversible atrophy, while atrophy induced by short-term use may to some extent be reversible except for striae.[3]
Corticosteroids are absorbed at different rates depending on the thickness of the stratum corneum. A mild topical steroid that works on the face may achieve little on the palm. But a potent steroid may quickly cause side effects on the face.
For example:
- Forearm absorbs 1%
- Armpit absorbs 4%
- Face absorbs 7%
- Eyelids and genitals absorb 30%
- Palm absorbs 0.1%
- Sole absorbs 0.05%[4]
Absorption is greater in an ointment base, in the presence of a keratolytic agent such as salicylic acid and under occlusion.[2] Strong topical steroid (e.g., fluorinated steroids) can induce the condition very quickly, while a weaker steroid can induce it slowly over time. Corticosteroid preparations that contain urea or salicylic acid are more potent than those containing the corticosteroid alone, as these ingredients increase the absorption of the steroid into the skin.[4]
Findings
General skin atrophy consists of a reduction in epidermal and dermal thickness, regression of the sebaceous glands, subcutaneous fat loss, and muscle-layer atrophy. These changes are typically observed following 2 to 3 weeks of moderate- to high-potency topical corticosteroid use. A single application of a very potent topical steroid can cause an ultrasonographically detectable decrease in skin thickness that lasts up to 3 days. Even low-potency topical steroids can cause slight skin atrophy that often reverses upon discontinuation of the drugs. Atrophy and striae are of concern on areas of the skin with high permeability, such as the face and intertriginous areas, but these adverse events can occur anywhere, especially after long-term use of moderate- or high-potency topical corticosteroids. While mild atrophy and telangiectasia might be reversible upon discontinuation of corticosteroids, overtly visible changes in skin texture and striae are considered permanent manifestations of corticosteroid-induced atrophy and are resistant to treatment.[5]
The therapeutic effects of topical steroids can be negated by the resulting thinning of the stratum corneum. Such thinning impairs its barrier function and allows transepidermal water loss that can lead to skin irritation.[6] Sometimes, the visible and textural changes to the skin are described as looking like “cigarette paper.” The skin thins because of decreased production of fibroblasts and abnormal deposition of collagen and elastin. Loss of hyaluronic acid leads to decreased retention of dermal moisture.[7]
The structural changes and the signs and symptoms of chronologically aged skin and those of corticosteroid induced chronic atrophy of the skin are partially very similar. Thinning of epidermis and laxity as well as dryness, purpura and echymoses occur in both conditions. However, in chronologically aged skin striae are not observed, while in corticosteroid atrophy premalignant or malignant tumours are seldom observed.[3]
Steroid Phobia
Recent medical studies have suggested that the fear of "skin thinning" is in many cases unfounded and can lead to poor compliance and treatment failure as a result of unnecessary fear. In most cases of topical corticosteroid when used appropriately and when supervised by a doctor there is little risk of skin thinning and serious adverse effects even with relatively long-term use (months).[8][9]
A recent study from 2011 states, "We conclude that routine, appropriate, long-term use of topocal corticosteroids (TCS) in children with dermatitis does not cause skin atrophy. These data do not support the widely held belief that routine use of TCS will "thin the skin." Parents, pharmacists, and health practitioners should be confident about the safety of using this treatment." [8]
Parental alternative health beliefs and fear of topical corticosteroids may lead to non-adherence and treatment failure. At the extreme end, such beliefs may result in neglect constituting reportable child maltreatment.[9]
Treatment
The obvious priority is immediate discontinuation of any further topical corticosteroid use. Protection and support of the impaired skin barrier is another priority. Eliminating harsh skin regimens or products will be necessary to minimize potential for further purpura or trauma, skin sensitivity, and potential infection. Steroid Atrophy[10][11] is often permanent, though if caught soon enough and the topical corticosteroid discontinued in time, the degree of damage may be arrested or slightly improve. However, while the accompanying Telangectasias may improve marginally, the Striae is permanent and irreversible.[1]
Recent studies have shown potential for treatment of permanent corticosteroid induced atrophy of the skin.[3][12]
Prevention
Judicious use of topical steroid, with strict attention to strength, area of application, duration and vehicle (e.g., ointments are more potent than creams. The occlusive nature of ointment enhances steroid penetration[13][14]). In general, use a potent preparation short term and weaker preparation for maintenance between flare-ups. While there is no proven best benefit-to-risk ratio,[15] if prolonged use of a topical steroid on a skin surface is required, a pulse therapy should be undertaken.
Pulse therapy refers to the application of a corticosteroid for 2 or 3 consecutive days each week or two. This is useful for maintaining control of chronic diseases. Generally a milder topical steroid or non-steroid treatment is used on the in-between days.[16]
Strong steroids should be avoided on sensitive sites such as the face, groin and armpits. Even the application of weaker or safer steroids should be limited to less than two weeks on those sites.[17]
Patients choosing to discontinue from topical steroid use should discuss this option with their doctor and visit supportive sites which promote safe steroid use and usage education.[18]
References
- 1 2 "Steroid Atrophy".
- 1 2 "Corticosteroids".
- 1 2 3 "The repairing effect of Vivida on skin atrophy induced by long-term use of potential topical corticosteroids".
- 1 2 "Topical Corticosteroids, Marshall, H. 6/11/2013".
- ↑ Ting, Patricia; Barankin, B (2006). "Atrophic patches". Canadian Family Physician. 52 (12): 1547, 1551–2. PMC 1783752
 . PMID 17279233.
. PMID 17279233. - ↑ Sarnstrand, Bengt; Brattsand, Ralph; Malmstrom, Anders (1982). "Effect of Glucocorticoids on Glycosaminoglycan Metabolism in Cultured Human Skin Fibroblasts". Journal of Investigative Dermatology. 79 (6): 412–7. doi:10.1111/1523-1747.ep12530360. PMID 7142743.
- ↑ Kolbe, Ludger; Kligman, Albert M.; Schreiner, Volker; Stoudemayer, Tracy (2001). "Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin". Skin Research and Technology. 7 (2): 73–7. doi:10.1034/j.1600-0846.2001.70203.x. PMID 11393207.
- 1 2 "Evaluation of the atrophogenic potential of topical corticosteroids in pediatric dermatology patients". PMID 21507057.
- 1 2 "Treatment failure in atopic dermatitis as a result of parental health belief". PMID 24099206.
- ↑ Fukaya, Mototsugu (2000). Color Atlas of Steroid Withdrawal from Corticosteroids in Patients with Atopic Dermatitis. Tokyo, Japan: Ishiyaku Publishers, Inc.
- ↑ Fukaya, Mototsugu (June 2000). Atopic Dermatitis and Steroid Withdrawal (1st ed.). Japan: Ishiyaku Pub,Inc. p. 107. ISBN 978-4-263-20140-4. (skin atrophy caused during application of the steroid ointment).
- ↑ Schwartz, E; Mezick, Ja; Gendimenico, Gj; Kligman, Lh (1995). "Treatment of steroid atrophy with topical tretinoin in the hairless mouse: A histologic, biochemical and immunochemical analysis". Journal of Dermatological Treatment. 6: 25. doi:10.3109/09546639509080586.
- ↑ "The Importance of the Topical Steroid Base".
- ↑ "Vehicle".
- ↑ "Choosing Topical Corticosteroids".
- ↑ "Course on topical steroids".
- ↑ "The long term side effects of steroids in topicals".
- ↑ "Topical Steroids 101".