Standard enthalpy of reaction
The standard enthalpy of reaction (denoted ΔHr⊖) is the enthalpy change that occurs in a system when one mole of matter is transformed by a chemical reaction under standard conditions.
For a generic chemical reaction
- −vA A + −vB B + ... → vP P + vQ Q ...
the standard enthalpy of reaction ΔHr⊖ is related to the standard enthalpy of formation ΔHfo of the reactants and products by the following equation:
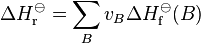
In this equation, vB is the stoichiometric number of entity B.
A similar enthalpy change is the standard enthalpy of formation, which has been determined for a vast number of substances. The enthalpy change of any reaction under any conditions can be computed, given the standard enthalpy of formation of the reactants and products.
It is defined as the amount of heat absorbed or evolved in the transformation of the reactants at a given temperature and pressure into the products at the same temperature and pressure. Enthalpy of a reaction at constant pressure and at a constant volume: Enthalpy of a reaction depends upon the conditions under which the reaction is carried out. There are two general conditions under which Thermochemical measurements are made.
(a) Constant volume (b) Constant pressure
The magnitudes of the enthalpy changes in these two conditions are different. In first case the volume of the system is kept constant during the course of the measurement by carrying out the reaction in a closed and rigid container and as there is no change in the volume and so no work is also involved.
From the first law of thermodynamics we have a relation, 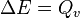
That is, the enthalpy of a reaction at constant volume is equal to the change in the internal energy (Δ E) of the reacting system.
The thermal change that occurs in a chemical reaction is only due to the difference in the sum of internal energy of the products and the sum of the internal energy of reactants.
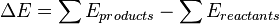
This also signifies that the amount of heat absorbed at constant volume could be identified with the change in the thermodynamic quantity.
At constant pressure, the system is either kept open to the atmosphere or confined within a container on which a constant external pressure is exerted and under these conditions the volume of the system changes. The thermal change at a constant pressure not only involves the change in the internal energy of the system but also the work performed either in expansion or contraction of the system.
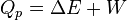 (work)
(work)
If ‘W’ is only pressure-volume work, then
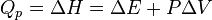
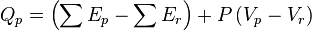
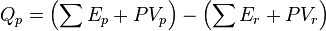
As enthalpy or heat content is defined by 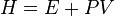 .
.
So we have, 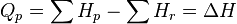
At constant pressure, the heat of the reaction is exactly equal to the enthalpy change, 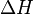 , of the reacting system.
, of the reacting system.