Batten disease
| Batten disease | |
|---|---|
| Spielmeyer-Vogt-Sjögren-Batten disease, Batten-Mayou disease, Vogt-Spielmeyer disease | |
| Classification and external resources | |
| Specialty | endocrinology |
| ICD-10 | E75.4 |
| ICD-9-CM | 330.1 |
| OMIM | 204200 |
| DiseasesDB | 31534 |
| MeSH | D009472 |
Batten disease is an extremely rare and fatal autosomal recessive neurodegenerative disorder that begins in childhood. It is the most common form of a group of disorders called the neuronal ceroid lipofuscinoses (NCLs).
Although Batten disease is usually regarded as the juvenile form of NCL (or "type 3"), some physicians use the term Batten disease to describe all forms of NCL. Historically, the NCLs were classified by age of disease onset as infantile NCL (INCL), late infantile NCL (LINCL), juvenile NCL (JNCL) or adult NCL (ANCL).[1]
At least twenty genes have been identified in association with Batten disease, but juvenile NCL, the most prevalent form of Batten disease, has been linked to mutations in the CLN3 gene.[2][3]
Signs and symptoms
Early signs and symptoms of the disorder usually appear around ages 2–10, with gradual onset of vision problems, or seizures. Early signs may be subtle personality and behavior changes, slow learning or regression, repetitive speech or echolalia, clumsiness, or stumbling. There may be slowing head growth in the infantile form, poor circulation in lower extremities (legs and feet), decreased body fat and muscle mass, curvature of the spine, hyperventilation and/or breath-holding spells, teeth grinding, and constipation.
Over time, affected children suffer mental impairment, worsening seizures, and progressive loss of sight, speech and motor skills. Eventually, children with Batten disease become blind, bedridden, demented, and die. Batten disease is a terminal disease; life expectancy varies depending on the type or variation.
Females with juvenile Batten disease show first symptoms a year later than males but on average die a year sooner.[4]
Cause
Neuronal ceroid lipofuscinoses (NCLs) are a family of diseases which are inherited in an autosomal recessive manner. Collectively referred to as Batten disease, the NCLs are responsible for the majority of neurodegnerative diseases that affect children. Specifically, the frequency of this disease is approximately 1 per 12,500 individuals. The specific type of NCL is characterized by the age of symptomatic onset and genetic mutation involved. Currently, it has been found that mutations in ten genes lead to the development Batten disease.[5]
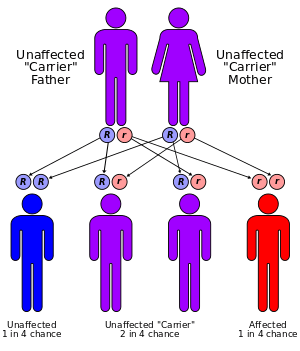
NCL diseases
- Infantile Neuronal Ceroid (INCL): CLN1 encodes for the protein PPT1 which functions as a lysosomal enzyme.[6]
- Late Infantile NCL (LINCL): CLN2 encodes for the protein TPP1 which serves as a lysosomal enzyme.[6]
- Juvenile NCL (JNCL): CLN3 encodes for CLN3, a lysosomal transmembrane protein.[6]
- Adult NCL: CLN4 has no known associated protein.[6]
- Finnish Variant of Late Infantile NCL (fLINCL): CLN5 encodes for CLN5, a soluble lysosomal protein.[6]
- Variant of the Late-Infantile NCL: CLN6 encodes for the protein CLN6, which serves as a transmembrane protein of the endoplasmic reticulum.[6]
- Turkish Variant of Late Infantile NCL: CLN7 or MFSD8, encodes for MFS8 which functions as a lysosomal transmembrane protein.[6]
- Northern Epilepsy: CLN8 encodes for CLN8, a transmembrane protein of the endoplasmic reticulum.[6]
- Late Infantile NCL: CLN10 or CTSD encodes for CTSD which is a lysosomal protein which a variety of functions.[6]
- Infantile Osteopetrosis: CLCN7 encodes for CLC7.[6]
Juvenile NCL (JNCL): CLN3 mutation
The CLN3 gene is located on the short arm of chromosome 16 at gene position 12.1 (16p12.1) and mutations within this gene are the major cause of juvenile NCL. More specifically, 73% of Batten disease cases are due to a 1.02 kb deletion within this gene, CLN3. This deletion causes a frameshift which produces a truncated mutant gene product of only 181 amino acids in length when compared to the wild-type gene product of 438 amino acids in length. Normal functioning CLN3 encodes for a hydrophobic transmembrane protein that is mainly localized to the lysosome; however, the 181 amino acid mutant gene product was instead found to primarily localize to the endoplasmic reticulum and Golgi apparatus. The precise function of the CLN3 gene product remains unknown.[5]
Diagnosis
Batten disease is a rare disease. Since it is a uncommon disease. Batten disease may result in misdiagnosis, which in turn cause increases in medical expenses, increases in family stress, and increases in the chance of using incorrect forms of treatment. Neverthless, Batten disease can be diagnosed if properly detected. Vision impairment is the most common observable symptom to detect the disease. Children are more prevalent, and should be suspected more for juvenile Batten disease.[7] Children or someone suspected to have Batten disease, should initially be seen by an optometrist or ophthalmologist. A fundus eye examination that aids in the detection of common vision impairment abnormalities, such as granularity of the retinal pigment epithelium in the central macula will be performed.[7] Even though it is also seen in a variety of other diseases as well, a loss of ocular cells should be a warning sign of Batten Disease potentially being present. If Batten Disease is the suspected diagnosis, a variety of tests are conducted to help accurately confirm its ascertainment including:
Blood or urine tests. Urinalysis and blood testing can help detect abnormalities that may indicate Batten disease. For example, elevated levels of dolichol in urine have been found in many individuals with NCL. The presence of vacuolated lymphocytes—white blood cells that contain holes or cavities (observed by microscopic analysis of blood smears)—when combined with other findings that indicate NCL, is suggestive for the juvenile form caused by CLN3 mutations.[8]
Skin or tissue sampling: Performed by extracting a small piece of tissue, which then is examined under an electron microscope. This helps and allows physicians to detect typical NCL deposits. These deposits are common in tissues, such as skin, muscle, conjunctiva, and rectal.[8] This type of diagnostic technique is beneficial, however other invasive tests are more advantageous for diagnosing Batten disease.
Electroencephalogram (EEG). EEG is a techinque that uses special probes that are attached on to the individuals scalp. It records electrical currents/signals, which allow medical experts to analylze electrical pattern activity in the brain. Assist in observing if the patient has seizures.[8]
Electrical studies of the eyes. As mentioned, vision loss is the most common characteristic of Batten disease. Visual-evoked responses and electroretinograms are effective test for detecting various eye conditions common in childhood NCLs.
Computed tomography (CT) or magnetic resonance imaging (MRI). Diagnostic imaging test allow physicians to better visualize the appearance of the brian. MRI imaging test uses magentic fields and radio waves to help create images of the brain. CT scan is another type of imaging test that uses x-rays and computers to create a detailed image of the brain's tissues and structures. Both diagnostic imaging test can help reveal brain areas that are decaying, or “atrophic,” in persons with NCL.[8]
Measurement of enzyme activity. Measuring enzymatic activity specific to Batten disease, may help confirm or rule out certain diagnoses caused by different mutations. Elevated levels of palmitoyl-protein thioesterase is involved in CLN1. Acid protease is involved in CLN2. Cathepsin D is involved in CLN10.[8]
DNA analysis. DNA analysis can be used to help confirm the diagnosis of Batten disease. When the mutation is known, DNA analysis can also be used to detect unaffected carriers of this condition for genetic counseling. If a family mutation has not previously been identified or if the common mutations are not present, recent molecular advances have made it possible to sequence all of the known NCL genes, increasing the chances of finding the responsible mutation(s).[8]
Treatment
Batten disease is a terminal illness, with no cure. Palliative treatment is symptomatic and supportive.
Research
In June 1987, a Phase I clinical trial was launched at Weill Medical College of Cornell University to study a gene therapy method for treatment of the signs and symptoms of late infantile neuronal ceroid lipofuscinosis (LINCL). The experimental drug works by delivering a gene transfer vector called AAV2CUhCLN2 to the brain.[9] Although the trial is not matched, randomized, or blinded and lacked a contemporaneous placebo/sham control group, assessment of the primary outcome variable suggests a slowing of progression of LINCL in the treated children.[10]
Researchers believe the neurological deficits common in JNCL could be due to overactive AMPA receptors in the cerebellum. To test this hypothesis, researchers administered AMPA antagonist drugs into affected mice. The motor skills of the affected mice showed significant improvement after the antagonist treatment, which supported the hypothesis that the neurological deficits in JNCL are due to overactive AMPA receptors. This research could eventually help to alleviate neurological deficits of JNCL in humans.[11]
In November 2006, after receiving FDA clearance, neurosurgeon Nathan Selden, pediatrician Bob Steiner, and colleagues at Doernbecher Children's Hospital at Oregon Health & Science University began a clinical study in which purified neural stem cells were injected into the brain of Daniel Kerner, a six-year-old child with Batten disease, who had lost the ability to walk and talk. This patient was the first of six to receive the injection of a stem cell product from StemCells Inc., a Palo Alto biotech company. These are believed to be the first-ever transplants of fetal stem cells into the human brain.[12] By early December, the child had recovered well enough to return home, and it was reported that there were some signs of speech returning.[13] Daniel Kerner died on April 12, 2010.[14] The main goal of Phase I clinical trials, however, was to investigate the safety of transplantation. Overall, the Phase I data demonstrated that high doses of human neural stem cells, delivered by a direct transplantation procedure into multiple sites within the brain, followed by twelve months of immunosuppression, were well tolerated by all six patients enrolled in the trial. The patients’ medical, neurological and neuropsychological conditions, following transplantation, appeared consistent with the normal course of the disease.[15]
Mycophenolate mofetil is being tested to determine its ability to safely slow or halt neurodegeneration.[16][17] A non-randomised safety and efficacy trial of a gene transfer vector is underway.[18]
History
Batten disease is named after the British pediatrician Frederick Batten, who first described it in 1903.[19][20] Also known as Spielmeyer-Vogt-Sjögren-Batten disease, it is the most common form of a group of disorders called neuronal ceroid lipofuscinosis (or NCLs). Although Batten disease is usually regarded as the juvenile form of NCL, some physicians use the term Batten disease to describe all forms of NCL.
See also
| Wikimedia Commons has media related to Batten disease. |
References
- ↑ Hobert JA, Dawson G (October 2006). "Neuronal ceroid lipofuscinoses therapeutic strategies: past, present and future". Biochimica et Biophysica Acta. 1762 (10): 945–53. doi:10.1016/j.bbadis.2006.08.004. PMID 17049436.
- ↑ Rakheja D, Narayan SB, Bennett MJ (September 2007). "Juvenile neuronal ceroid-lipofuscinosis (Batten disease): a brief review and update". Current Molecular Medicine. 7 (6): 603–8. doi:10.2174/156652407781695729. PMID 17896996.
- ↑ Cooper JD (June 2008). "Moving towards therapies for juvenile Batten disease?". Experimental Neurology. 211 (2): 329–31. doi:10.1016/j.expneurol.2008.02.016. PMID 18400221.
- ↑ Cialone J, Adams H, Augustine EF, et al. (May 2012). "Females experience a more severe disease course in Batten disease". Journal of Inherited Metabolic Disease. 35 (3): 549–55. doi:10.1007/s10545-011-9421-6. PMC 3320704
 . PMID 22167274.
. PMID 22167274. - 1 2 Jill M. Weimer, Elizabeth Kriscenski-Perry, Yasser Elshatory, David A. Pearce (2002). "The Neuronal Ceroid Lipofuscinoses: Mutations in Different Proteins Result in Similar Disease". NeuroMolecular Medicine. 1: 111–124.
- 1 2 3 4 5 6 7 8 9 10 Jalanko Anu, Braulke Thomas (2009). "Neuronal ceroid lipofuscinoses". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1793: 697–709.
- 1 2 Ostergaard, John R (2016-08-01). "Juvenile neuronal ceroid lipofuscinosis (Batten disease): current insights". Degenerative Neurological and Neuromuscular Disease. 6. doi:10.2147/DNND.S111967.
- 1 2 3 4 5 6 "Noah's Hope - Causes and Symptoms of Batten Disease". www.noahshope.com. Retrieved 2016-11-22.
- ↑ Clinical trial number NCT00151216 for "Safety Study of a Gene Transfer Vector for Children With Late Infantile Neuronal Ceroid Lipofuscinosis" at ClinicalTrials.gov
- ↑ Worgall S, Sondhi D, Hackett NR, et al. (May 2008). "Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA". Human Gene Therapy. 19 (5): 463–74. doi:10.1089/hum.2008.022. PMID 18473686.
- ↑ Kovács, Attila D.; Pearce, David A. (2008-01-01). "Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease". Experimental Neurology. The Role of α-synuclein in the Pathogenesis of Parkinson's Disease / Gene Therapy for Parkinson's. 209 (1): 288–291. doi:10.1016/j.expneurol.2007.09.012. PMC 4418195
 . PMID 17963751.
. PMID 17963751. - ↑ "A stem cell first at OHSU" The Portland Tribune, Nov 24, 2006
- ↑ http://www.technologyreview.com/read_article.aspx?id=17888&ch=biotech[]
- ↑ http://writethehappyending.com/tag/daniel-kerner/
- ↑ "StemCells: Enter Date".
- ↑ http://www.medicalnewstoday.com/releases/227090.php[]
- ↑ Clinical trial number NCT01399047 for "Cellcept for Treatment of Juvenile Neuronal Ceroid Lipofuscinosis (JUMP)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01161576 for "Safety Study of a Gene Transfer Vector (Rh.10) for Children With Late Infantile Neuronal Ceroid Lipofuscinosis" at ClinicalTrials.gov
- ↑ synd/7 at Who Named It?
- ↑ Batten FE (1902). "Cerebral degeneration with symmetrical changes in the maculae in two members of a family". Transactions of the Ophthalmological Societies of the United Kingdom. 23: 386–90.
External links
- http://bdfa-uk.org.uk UK Batten Disease Family Association
- http://beyondbatten.org
- Batten disease at University of Rochester Medical Centre
- Batten Disease at NINDS
- GeneReviews/NCBI/NIH/UW entry on Neuronal Ceroid-Lipofuscinosis
- About Batten Disease
- NCL resource - Batten disease at University College London
- Batten Disease Support and Research Association (bdsra.org)
- What is Batten? at lrbf.org
- Batten Disease Support and Research Organisation (Australia)
- Batten FE, Mayou MS (1915). "Family Cerebral Degeneration with Macular Changes". Proceedings of the Royal Society of Medicine. 8 (Sect Ophthalmol): 70–90. PMC 2003604
 . PMID 19978990.
. PMID 19978990. - Living with Batten Disease on YouTube