Sodium hypophosphite
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium phosphinate | |
| Identifiers | |
| 7681-53-0 10039-56-2 (monohydrate) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 21241942 |
| ECHA InfoCard | 100.028.791 |
| PubChem | 16129646 |
| RTECS number | SZ5640000 (monohydrate) |
| |
| |
| Properties | |
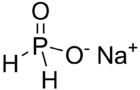
| NaPO2H2 | |
| Molar mass | 87.98 g/mol (anhydrous) 105.99 g/mol (monohydrate) |
| Appearance | white solid |
| Density | 0.8 g/cm3 (monohydrate) |
| Melting point | 90 °C (194 °F; 363 K) (monohydrate) |
| soluble | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions |
Sodium phosphite Monosodium phosphate Disodium phosphate Trisodium phosphate |
| Other cations |
Potassium hypophosphite |
| Related compounds |
Hypophosphorous acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium hypophosphite (NaPO2H2, also known as sodium phosphinate) is the sodium salt of hypophosphorous acid and is often encountered as the monohydrate, NaPO2H2·H2O. It is a solid at room temperature, appearing as odorless white crystals. It is soluble in water, and easily absorbs moisture from the air.
Sodium hypophosphite should be kept in a cool, dry place, isolated from oxidizing materials. It decomposes when heated and produces toxic phosphine gas, causing irritation to the respiratory tract.
- 2 NaH2PO2 → Na2HPO4 + PH3
Uses
Sodium hypophosphite is mainly used for electroless nickel plating (Ni-P).[1] With this method, a durable nickel-phosphorus film can coat objects with irregular surfaces, and can widely be in avionics, aviation and the petroleum field.
Sodium hypophosphite is capable of reducing nickel ions in solution to metallic nickel on metal substrates as well as on plastic substrates.[2] The latter requires that the substrate is activated with fine particles of palladium. The resulting nickel deposit contains up to 15% phosphorus.
It also can be used as a food additive.
DEA List I status
The United States Drug Enforcement Administration designated sodium hypophosphite as a List I chemical under 21 CFR 1310.02 effective November 17, 2001, specifically mentioning the compound together with several other salts of hypophosphorous acid.[3][4]
References
- ↑ Abrantes, L. M. (1994). "On the Mechanism of Electroless Ni-P Plating". Journal of The Electrochemical Society. 141 (9): 2356. doi:10.1149/1.2055125.
- ↑ D. Rich & M. Smith, Electroless Deposition of Nickel, Cobalt and Iron, IBM Corp (1971)
- ↑ 66 FR 52670—52675. 17 October 2001.
- ↑ 37 CFR 1310.02