Simmons–Smith reaction
 Simmons-Smith reaction in progress |
| Simmons-Smith reaction | |
|---|---|
| Named after | Howard Ensign Simmons, Jr. Ronald D. Smith |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | simmons-smith-reaction |
| RSC ontology ID | RXNO:0000258 |
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene (or alkyne) to form a cyclopropane.[1][2][3] It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.[4]
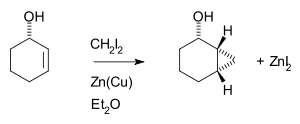
Thus, cyclohexene, diiodomethane, and a zinc-copper couple (as iodomethylzinc iodide, ICH2ZnI) yield norcarane (bicyclo[4.1.0]heptane).[5][6]
The Simmons–Smith reaction is generally preferred over other methods of cyclopropanation,[7] however it can be expensive due to the high cost of diiodomethane. Modifications involving cheaper alternatives have been developed, such as dibromomethane[8] or diazomethane and zinc iodide.[9] The reactivity of the system can also be increased by exchanging the zinc‑copper couple for diethylzinc,[10] however as this reagent is pyrophoric it must be handled carefully.
The Simmons–Smith reaction is generally subject to steric effects, and thus cyclopropanation usually takes place on the less hindered face.[11][12] However, when a hydroxy substituent is present in the substrate in proximity to the double bond, the zinc coordinates with the hydroxy substituent, directing cyclopropanation cis to the hydroxyl group (which may not correspond to cyclopropanation of the sterically most accessible face of the double bond):[13] An interactive 3D model of this reaction can be seen here (java required).
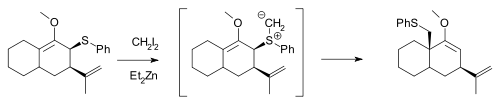
The Simmons–Smith reagent, namely diiodomethane and diethylzinc, can react with allylic thioethers to generate sulfur ylides, which can subsequently undergo a 2,3-sigmatropic rearrangement, and will not cyclopropanate an alkene in the same molecule unless excess Simmons–Smith reagent is used:[14]
Asymmetric Simmons–Smith reaction
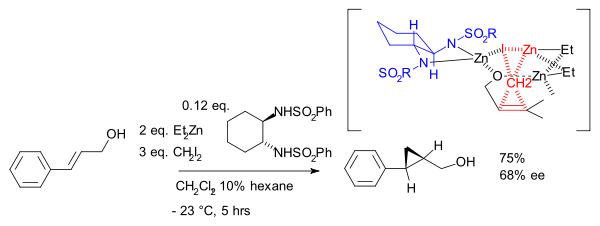
Although asymmetric cyclopropanation methods based on diazo compounds (see bisoxazoline ligand) exist since 1966, the asymmetric Simmons–Smith reaction was introduced in 1992 [15] with a reaction of cinnamyl alcohol with diethylzinc, diiodomethane and a chiral disulfonamide in dichloromethane:
The hydroxyl group is a prerequisite serving as an anchor for zinc. An interactive 3D model of a similar reaction[16] can be seen here (java required). In another version of this reaction the ligand is based on salen and Lewis acid DIBAL is added:[17]
References
- ↑ Howard Ensign Simmons, Jr.; Smith, R.D. (1958). "A New Synthesis of Cyclopropanes from Olefins". J. Am. Chem. Soc. 80: 5323. doi:10.1021/ja01552a080.
- ↑ Simmons, H.E.; Smith, R.D. (1959). "A New Synthesis of Cyclopropanes". J. Am. Chem. Soc. 81: 4256. doi:10.1021/ja01525a036.
- ↑ Denis, J.M.; Girard, J.M.; Conia, J.M (1972). "Improved Simmons–Smith Reactions". Synthesis. 1972: 549. doi:10.1055/s-1972-21919.
- ↑ Charette, A. B.; Beauchemin, A. Org. React. 2001, 58, 1. doi:10.1002/0471264180.or058.01
- ↑ Smith, R. D.; Simmons, H. E. "Norcarane". Org. Synth.; Coll. Vol., 5, p. 855
- ↑ Ito, Y.; Fujii, S.; Nakatuska, M.; Kawamoto, F.; Saegusa, T. (1988). "One-Carbon Ring Expansion Of Cycloalkanones To Conjugated Cycloalkenones: 2-Cyclohepten-1-one". Org. Synth.; Coll. Vol., 6, p. 327
- ↑ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. ISBN 978-0-19-850346-0.Page 1067
- ↑ Fabisch, Bodo; Mitchell, Terence N. (1984). "An inexpensive modification of the Simmons-Smith reaction: The formation of bromomethylzinc bromide as studied by NMR spectroscopy". Journal of Organometallic Chemistry. 269 (3): 219–221. doi:10.1016/0022-328X(84)80305-8.
- ↑ Wittig, Georg; Wingler, Frank (1 August 1964). "Über methylenierte Metallhalogenide, IV. Cyclopropan-Bildung aus Olefinen mit Bis-halogenmethyl-zink". Chemische Berichte. 97 (8): 2146–2164. doi:10.1002/cber.19640970808.
- ↑ Furukawa, J.; Kawabata, N.; Nishimura, J. (1968). "Synthesis of cyclopropanes by the reaction of olefins with dialkylzinc and methylene iodide". Tetrahedron. 24 (1): 53–58. doi:10.1016/0040-4020(68)89007-6.
- ↑ Simmons, H. E.; et al. (1973). Org. React. (Review). 20: 1. Missing or empty
|title=(help) - ↑ Girard, C.; Conia, J. M. (1978). J. Chem. Res. (S) (Review): 182. Missing or empty
|title=(help) - ↑ Paul A. Grieco; Tomei Oguri; Chia-Lin J. Wang & Eric Williams (1977). "Stereochemistry and total synthesis of (±)-ivangulin". J. Org. Chem. 42: 4113. doi:10.1021/jo00445a027.
- ↑ Cohen, T.; Kosarych, Z. (1982). "Complete regio- and stereospecificity in the Lewis acid catalyzed Diels-Alder reactions of (Z)-2-methoxy-1-(phenylthio)-1,3-butadienes. Conversion of the CS configuration of an adduct to the CC configuration at the allylic position by a [2,3] sigmatropic rearrangement". J. Org. Chem. 47: 4005. doi:10.1021/jo00141a047.
- ↑ Hideyo Takahashi, Masato Yoshioka, Masaji Ohno and Susumu Kobayashi (1992). "A catalytic enantioselective reaction using a C2-symmetric disulfonamide as a chiral ligand: cyclopropanation of allylic alcohols by the Et2Zn-CH2I2-disulfonamide system". Tetrahedron Letters. 33 (18): 2575–2578. doi:10.1016/S0040-4039(00)92246-9.
- ↑ Wang, Tao; Liang, Yong; Yu, Zhi-Xiang (2011). "Density Functional Theory Study of the Mechanism and Origins of Stereoselectivity in the Asymmetric Simmons–Smith Cyclopropanation with Charette Chiral Dioxaborolane Ligand". Journal of the American Chemical Society. 133 (24): 9343–9353. doi:10.1021/ja111330z.
- ↑ Hiroaki Shitama & Tsutomu Katsuki (2008). "Asymmetric Simmons–Smith Reaction of Allylic Alcohols with Al Lewis Acid/N Lewis Base Bifunctional Al(Salalen) Catalyst". Angew. Chem. Int. Ed. 47: 2450. doi:10.1002/anie.200705641.
External links
| Wikimedia Commons has media related to Simmons-Smith reaction. |