Seliwanoff's test

Seliwanoff’s test is a chemical test which distinguishes between aldose and ketose sugars. Ketoses are distinguished from aldoses via their ketone/aldehyde functionality. If the sugar contains a ketone group, it is a ketose. If a sugar contains an aldehyde group, it is an aldose. This test relies on the principle that, when heated, ketoses are more rapidly dehydrated than aldoses. It is named after Theodor Seliwanoff, the chemist that devised the test. When added to a solution containing ketoses, a red color is formed rapidly indicating a positive test. When added to a solution containing aldoses, a slower forming light pink is observed instead.
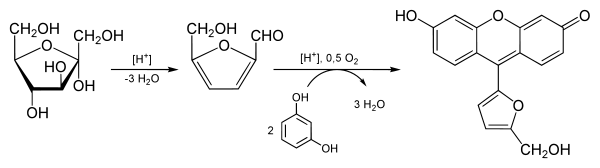
The reagents consist of resorcinol and concentrated hydrochloric acid:
- The acid hydrolysis of polysaccharide and oligosaccharide ketoses yields simpler sugars followed by furfural.[1]
- The dehydrated ketose then reacts with two equivalents of resorcinol in a series of condensation reactions to produce a molecule with a deep cherry red color.
- Aldoses may react slightly to produce a faint pink color.
Fructose and sucrose are two common sugars which give a positive test. Sucrose gives a positive test as it is a disaccharide consisting of fructose and glucose.
References
- ↑ Abramoff, Peter; Thomson, Robert (1966). An experimental approach to biology. WH Freeman & Company, San Francisco. p. 47.
- Seliwanoff, Theodor (1887). "Notiz über eine Fruchtzuckerreaction". Berichte der deutschen chemischen Gesellschaft. 20: 181. doi:10.1002/cber.18870200144.
- Katoch, Rajan (2011-06-30). Analytical Techniques in Biochemistry and Molecular Biology. p. 71. ISBN 978-1-4419-9784-5.
- Chawla (2003-01-01). Practical Clinical Biochemistry: Methods and Interpretations. p. 35. ISBN 978-81-8061-108-7.