Sunscreen
| Sunscreen | |
|---|---|
| Intervention | |
 Sunscreen on back under normal and UV light | |
| Synonyms | sun screen, sunblock, suntan lotion, sunburn cream, sun cream, block out[1] |
Sunscreen, also known as suncream, is a lotion, spray, gel or other topical product that absorbs or reflects some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn. Skin-lightening products have sunscreen to protect lightened skin because light skin is more susceptible to sun damage than darker skin. A number of sunscreens have tanning powder to help the skin to darken or tan; however, tanning powder does not provide protection from UV rays.
Depending on the mode of action, sunscreens can be classified into physical sunscreens (i.e., those that reflect the sunlight) or chemical sunscreens (i.e., those that absorb the UV light).[2]
Medical organizations such as the American Cancer Society recommend the use of sunscreen because it aids in the prevention of squamous cell carcinomas.[3] Many sunscreens do not block UVA radiation, which does not primarily cause sunburn but can increase the rate of melanoma and photodermatitis.[4] The use of broad-spectrum (UVA/UVB) sunscreens can address this concern. Diligent use of sunscreen can also slow or temporarily prevent the development of wrinkles and sagging skin.[5]
Sunscreens are commonly rated and labeled with a sun protection factor (SPF) that measures the fraction of sunburn-producing UV rays that reach the skin. For example, "SPF 15" means that 1/15th of the burning radiation reaches the skin through the recommended thickness of sunscreen. Other rating systems indicate the degree of protection from non-burning UVA radiation.
Health effects
Benefits
Sunscreen use can help prevent melanoma[6][7][8] and squamous cell carcinoma, two types of skin cancer.[9] There is little evidence that it is effective in preventing basal cell carcinoma.[10]
A 2013 study concluded that the diligent, everyday application of sunscreen can slow or temporarily prevent the development of wrinkles and sagging skin. The study involved 900 white people in Australia and required some of them to apply a broad-spectrum sunscreen every day for four and a half years. It found that people who did so had noticeably more resilient and smoother skin than those assigned to continue their usual practices.[5]
Minimizing UV damage is especially important for children and fair-skinned individuals and those who have sun sensitivity for medical reasons.[11]
Potential risks
In 2009, the Therapeutic Goods Administration of Australia updated a review of sunscreen safety studies and concluded: "The potential for titanium dioxide (TiO2) and zinc oxide (ZnO) nanoparticles in sunscreens to cause adverse effects depend primarily upon the ability of the nanoparticles to reach viable skin cells. To date, the current weight of evidence suggests that TiO2 and ZnO nanoparticles do not reach viable skin cells."[12] Sunscreen ingredients typically undergo testing by government regulators, and ingredients that present significant safety concerns (such as PABA) tend to be withdrawn from the consumer market.
Concerns have also been raised about potential vitamin D deficiency arising from prolonged use of sunscreen. Typical use of sunscreen does not usually result in vitamin D deficiency; however, extensive usage may.[13] Sunscreen prevents ultraviolet light from reaching the skin, and even moderate protection can substantially reduce vitamin D synthesis.[14][15] However, adequate amounts of vitamin D can be produced with moderate sun exposure to the face, arms and legs, averaging 5–30 minutes twice per week without sunscreen. (The darker the complexion, or the weaker the sunlight, the more minutes of exposure are needed, approximating 25% of the time for minimal sunburn. Vitamin D overdose is impossible from UV exposure; the skin reaches an equilibrium where the vitamin degrades as fast as it is created.)[16][17][18]
History
Early civilizations used a variety of plant products to help protect the skin from sun damage. For example, ancient Greeks used olive oil for this purpose, and ancient Egyptians used extracts of rice, jasmine, and lupine plants whose products are still used in skin care today.[19] Zinc oxide paste has also been popular for skin protection for thousands of years.[20][21]
Early synthetic sunscreens were first used in 1928, and the first major commercial product was brought to market in 1936, introduced by the founder of L'Oreal, French chemist Eugène Schueller. During the same period, Hamilton Sunscreen came to the Australian market in 1932, developed by chemist H. A. Milton Blake.[19][22]
Among widely used modern sunscreens, one of the earliest was produced in 1944 for the US military by Benjamin Green, an airman and later a pharmacist, as the hazards of sun overexposure became apparent to soldiers in the Pacific tropics at the height of World War II.[22][23][24][25] The product, named Red Vet Pet (for red veterinary petrolatum), had limited effectiveness, working as a physical blocker of ultraviolet radiation. It was a disagreeable red, sticky substance similar to petroleum jelly. Sales boomed when Coppertone improved and commercialized the substance under the Coppertone girl and Bain de Soleil branding in the early 1950s.
In 1946, Swiss chemist Franz Greiter introduced what may have been the first effective modern sunscreen. The product, called Gletscher Crème (Glacier Cream), subsequently became the basis for the company Piz Buin, which is still today a marketer of sunscreen products, named in honor of the mountain where Greiter allegedly obtained the sunburn that inspired his concoction.[19][26][27] In 1974, Greiter adapted earlier calculations from Friedrich Ellinger and Rudolf Schulze and introduced the "sun protection factor" (SPF), which has become a worldwide standard for measuring the effectiveness of sunscreen.[24][28] It has been estimated that Gletscher Crème had an SPF of 2.
Water-resistant sunscreens were introduced in 1977,[22] and recent development efforts have focused on making sunscreen protection both longer-lasting and broader-spectrum, as well as more appealing to use.[24]
Measurements of protection

Sun protection factor and labeling

The SPF rating is a measure of the fraction of sunburn-producing UV rays that reach the skin. For example, "SPF 15" means that 1/15th of the burning radiation will reach the skin, assuming sunscreen is applied evenly at a thick dosage of 2 milligrams per square centimeter (mg/cm2). A user can determine the effectiveness of a sunscreen "by multiplying the SPF factor by the length of time it takes for him or her to suffer a burn without sunscreen."[29] Thus, if a person develops a sunburn in 10 minutes when not wearing a sunscreen, the same person in the same intensity of sunlight will avoid sunburn for 150 minutes if wearing a sunscreen with an SPF of 15.[29] It is important to note that sunscreens with higher SPF do not last or remain effective on the skin any longer than lower SPF and must be continually reapplied as directed, usually every two hours.[30]
The SPF is an imperfect measure of skin damage because invisible damage and skin aging are also caused by ultraviolet type A (UVA, wavelengths 315–400 or 320–400 nm), which does not primarily cause reddening or pain. Conventional sunscreen blocks very little UVA radiation relative to the nominal SPF; broad-spectrum sunscreens are designed to protect against both UVB and UVA.[31][32][33] According to a 2004 study, UVA also causes DNA damage to cells deep within the skin, increasing the risk of malignant melanomas.[34] Even some products labeled "broad-spectrum UVA/UVB protection" have not always provided good protection against UVA rays.[35] Titanium dioxide probably gives good protection, but does not completely cover the UVA spectrum, as early 2000s research suggests that zinc oxide is superior to titanium dioxide at wavelengths 340–380 nm.[36]
Owing to consumer confusion over the real degree and duration of protection offered, labeling restrictions are enforced in several countries. In the EU, sunscreen labels can only go up to SPF 50+ (initially listed as 30 but soon revised to 50).[37] Australia's Therapeutic Goods Administration increased the upper limit to 50+ in 2012.[38][39] In its 2007 and 2011 draft rules, the US Food and Drug Administration (FDA) proposed a maximum SPF label of 50, to limit unrealistic claims.[40][41][42] (As of May 2016, the FDA has not adopted the SPF 50 limit.) Others have proposed restricting the active ingredients to an SPF of no more than 50, due to lack of evidence that higher dosages provide more meaningful protection.[43]
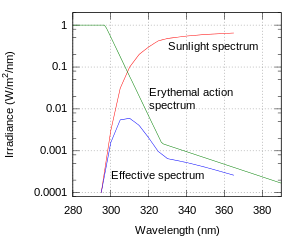
The SPF can be measured by applying sunscreen to the skin of a volunteer and measuring how long it takes before sunburn occurs when exposed to an artificial sunlight source. In the US, such an in vivo test is required by the FDA. It can also be measured in vitro with the help of a specially designed spectrometer. In this case, the actual transmittance of the sunscreen is measured, along with the degradation of the product due to being exposed to sunlight. In this case, the transmittance of the sunscreen must be measured over all wavelengths in sunlight's UVB–UVA range (290–400 nm), along with a table of how effective various wavelengths are in causing sunburn (the erythemal action spectrum) and the standard intensity spectrum of sunlight (see the figure). Such in vitro measurements agree very well with in vivo measurements .
Numerous methods have been devised for evaluation of UVA and UVB protection. The most-reliable spectrophotochemical methods eliminate the subjective nature of grading erythema.[44]
The ultraviolet protection factor (UPF) is a similar scale developed for rating fabrics for sun protective clothing. According to recent testing by Consumer Reports, UPF ~30 is typical for protective fabrics, while UPF ~6 is typical for standard summer fabrics.[45]
Mathematically, the SPF (or the UPF) is calculated from measured data as
where is the solar irradiance spectrum, the erythemal action spectrum, and the monochromatic protection factor, all functions of the wavelength . The MPF is roughly the inverse of the transmittance at a given wavelength.
The above means that the SPF is not simply the inverse of the transmittance in the UVB region. If that were true, then applying two layers of SPF 5 sunscreen would always be equivalent to SPF 25 (5 times 5). The actual combined SPF may be lower than the square of the single-layer SPF.[46]
UVA protection
Persistent pigment darkening
The persistent pigment darkening (PPD) method is a method of measuring UVA protection, similar to the SPF method of measuring sunburn protection. Originally developed in Japan, it is the preferred method used by manufacturers such as L'Oréal.
Instead of measuring erythema or reddening of the skin, the PPD method uses UVA radiation to cause a persistent darkening or tanning of the skin. Theoretically, a sunscreen with a PPD rating of 10 should allow a person 10 times as much UVA exposure as would be without protection. The PPD method is an in vivo test like SPF. In addition, Colipa has introduced a method that, it is claimed, can measure this in vitro and provide parity with the PPD method.[47]
SPF equivalence

As part of revised guidelines for sunscreens in the EU, there is a requirement to provide the consumer with a minimum level of UVA protection in relation to the SPF. This should be a "UVA PF" of at least 1/3 of the SPF to carry the UVA seal.[48]
A set of final US FDA rules effective from summer 2012 defines the phrase "broad spectrum" as providing UVA protection proportional to the UVB protection, using a standardized testing method.[41]
Star rating system
In the UK and Ireland, the Boots star rating system is a proprietary in vitro method used to describe the ratio of UVA to UVB protection offered by sunscreen creams and sprays. Based on original work by Brian Diffey at Newcastle University, the Boots Company in Nottingham, UK, developed a method that has been widely adopted by companies marketing these products in the UK.[49]
One-star products provide the lowest ratio of UVA protection, five-star products the highest. The method was recently revised in light of the Colipa UVA PF test and the revised EU recommendations regarding UVA PF. The method still uses a spectrophotometer to measure absorption of UVA versus UVB; the difference stems from a requirement to pre-irradiate samples (where this was not previously required) to give a better indication of UVA protection and photostability when the product is used. With the current methodology, the lowest rating is three stars, the highest being five stars.
In August 2007, the FDA put out for consultation the proposal that a version of this protocol be used to inform users of American product of the protection that it gives against UVA;[40] but this was not adopted, for fear it would be too confusing.[43]
PA system
Asian brands, particularly Japanese ones, tend to use The Protection Grade of UVA (PA) system to measure the UVA protection that a sunscreen provides. The PA system is based on the PPD reaction and is now widely adopted on the labels of sunscreens. According to the Japan Cosmetic Industry Association, PA+ corresponds to a UVA protection factor between two and four, PA++ between four and eight, and PA+++ more than eight.
Sunblock
Sunblock typically refers to opaque sunscreen that is effective at blocking both UVA and UVB rays and uses a heavy carrier oil to resist being washed off. Titanium dioxide and zinc oxide are two of the important ingredients in sunblock.[50] Unlike the organic sun-blocking agents used in many sunscreens, these metal oxides do not degrade with exposure to sunlight.
The use of the word "sunblock" in the marketing of sunscreens is controversial. Since 2013, the FDA has banned such use because it can lead consumers to overestimate the effectiveness of products so labeled.[41] Nonetheless, many consumers use the words sunblock and sunscreen synonymously.
For total protection against damage from the sun, the skin needs to be protected from UVA, UVB, and also IRA (infrared-A light).[51] Roughly 35% of solar energy is IRA.
Active ingredients
Sunscreens contain one or more of the following ingredients, which are either chemical or mineral in nature:
- Organic chemical compounds that absorb ultraviolet light.
- Inorganic particulates that reflect, scatter, and absorb UV light (such as titanium dioxide, zinc oxide, or a combination of both).[50]
- Organic particulates that mostly absorb light like organic chemical compounds, but contain multiple chromophores that may reflect and scatter a fraction of light like inorganic particulates, and behave differently in formulations than organic chemical compounds. An example is Tinosorb M. Since the UV-attenuating efficacy depends strongly on particle size, the material is micronised to particle sizes below 200 nm. The mode of action of this photostable filter system is governed to about 90% by absorption and 10% by scattering of UV light.
The principal ingredients in sunscreens are usually aromatic molecules conjugated with carbonyl groups. This general structure allows the molecule to absorb high-energy ultraviolet rays and release the energy as lower-energy rays, thereby preventing the skin-damaging ultraviolet rays from reaching the skin. So, upon exposure to UV light, most of the ingredients (with the notable exception of avobenzone) do not undergo significant chemical change, allowing these ingredients to retain the UV-absorbing potency without significant photodegradation.[52] A chemical stabilizer is included in some sunscreens containing avobenzone to slow its breakdown; examples include formulations containing Helioplex[53] and AvoTriplex.[54] The stability of avobenzone can also be improved by bemotrizinol,[55] octocrylene[56] and various other photostabilisers. Most organic compounds in sunscreens slowly degrade and become less effective over the course of several years if stored properly, resulting in the expiration dates calculated for the product.[57]
Sunscreening agents are used in some hair care products such as shampoos, conditioners and styling agents to protect against protein degradation and color loss. Currently, benzophenone-4 and ethylhexyl methoxycinnamate are the two sunscreens most commonly used in hair products. The common sunscreens used on skin are rarely used for hair products due to their texture and weight effects.
The following are the FDA allowable active ingredients in sunscreens:
| UV-filter | Other names | Maximum concentration | Permitted in these countries | Results of safety testing | UVA | UVB |
|---|---|---|---|---|---|---|
| p-Aminobenzoic acid | PABA | 15% (EU: banned from sale to consumers from 8 October 2009) | USA, AUS | Protects against skin tumors in mice.[58][59][60] Shown to increase DNA defects, however, and is now less commonly used. | X | |
| Padimate O | OD-PABA, octyldimethyl-PABA, σ-PABA | 8% (EU, USA, AUS) 10% (JP)
(Not currently supported in EU and may be delisted) |
EU, USA, AUS, JP | X | ||
| Phenylbenzimidazole sulfonic acid | Ensulizole, Eusolex 232, PBSA, Parsol HS | 4% (US, AUS) 8% (EU) 3% (JP) | EU, USA, AUS, JP | Genotoxic in bacteria[61] | X | |
| Cinoxate | 2-Ethoxyethyl p-methoxycinnamate | 3% (US) 6% (AUS) | USA, AUS | X | X | |
| Dioxybenzone | Benzophenone-8 | 3% | USA, AUS | X | X | |
| Oxybenzone | Benzophenone-3, Eusolex 4360, Escalol 567 | 6% (US) 10% (AUS, EU) 5% (JP) | EU, USA, AUS, JP | X | X | |
| Homosalate | Homomethyl salicylate, HMS | 10% (EU, JP) 15% (US, AUS) | EU, USA, AUS, JP | X | ||
| Menthyl anthranilate | Meradimate | 5% | USA, AUS | X | ||
| Octocrylene | Eusolex OCR, 2-Cyano-3,3-diphenyl acrylic acid, 2-ethylhexylester | 10% | EU, USA, AUS, JP | Increases ROS[62] | X | X |
| Octyl methoxycinnamate | Octinoxate, EMC, OMC, Ethylhexyl methoxycinnamate, Escalol 557, 2-Ethylhexyl-paramethoxycinnamate, Parsol MCX | 7.5% (US) 10% (EU, AUS) 20% (JP) | EU, USA, AUS, JP | X | ||
| Octyl salicylate | Octisalate, 2-Ethylhexyl salicylate, Escalol 587, | 5% (EU, USA, AUS) 10% (JP) | EU, USA, AUS, JP | X | ||
| Sulisobenzone | 2-Hydroxy-4-Methoxybenzophenone-5-sulfonic acid, 3-Benzoyl-4-hydroxy-6-methoxybenzenesulfonic acid, Benzophenone-4, Escalol 577 | 5% (EU) 10% (US, AUS, JP) | EU, USA, AUS, JP | X | X | |
| Trolamine salicylate | Triethanolamine salicylate | 12% | USA, AUS | X | ||
| Avobenzone | 1-(4-methoxyphenyl)-3-(4-tert-butyl phenyl)propane-1,3-dione, Butyl methoxy dibenzoylmethane, BMDBM, Parsol 1789, Eusolex 9020 |
3% (US) 5% (EU, AUS) 10% (JP) | EU, USA, AUS, JP | Not available[63] | X | |
| Ecamsule | Mexoryl SX, Terephthalylidene Dicamphor Sulfonic Acid | 10% | EU, AUS (US: approved in certain formulations up to 3% via New Drug Application (NDA) Route) | Protects against skin tumors in mice[64][65][66] | X | |
| Titanium dioxide | CI77891 | 25% (US) No limit (JP) | EU, USA, AUS, JP | X | X | |
| Zinc oxide | 25% (US) No limit (AUS, JP) | USA, AUS, JP | Protects against skin tumors in mice[64] | X | X |
Zinc oxide is not approved as a UV filter under Article 14 of the EU's 2009 regulation on cosmetic products.[67] However, it is approved and widely used as a colorant, and the Scientific Committee on Consumer Safety has issued favorable opinions for future approval as a UV filter.[68]
Other ingredients approved within the EU[67] and other parts of the world,[69] that have not been included in the current FDA Monograph:
| UV-filter | Other names | Maximum concentration | Permitted in |
|---|---|---|---|
| 4-Methylbenzylidene camphor | Enzacamene, Parsol 5000, Eusolex 6300, MBC | 4%* | EU, AUS |
| Tinosorb M | Bisoctrizole, Methylene Bis-Benzotriazolyl Tetramethylbutylphenol, MBBT | 10%* | EU, AUS, JP |
| Tinosorb S | Bis-ethylhexyloxyphenol methoxyphenol triazine, Bemotrizinol, BEMT, anisotriazine | 10% (EU, AUS) 3% (JP)* | EU, AUS, JP |
| Tinosorb A2B | Tris-Biphenyl Triazine | 10% | EU |
| Neo Heliopan AP | Bisdisulizole Disodium, Disodium phenyl dibenzimidazole tetrasulfonate, bisimidazylate, DPDT | 10% | EU, AUS |
| Mexoryl XL | Drometrizole Trisiloxane | 15% | EU, AUS |
| Benzophenone-9 | Uvinul DS 49, CAS 3121-60-6, Sodium Dihydroxy Dimethoxy Disulfobenzophenone [70] | 10% | JP |
| Uvinul T 150 | Octyl triazone, ethylhexyl triazone, EHT | 5% (EU, AUS) 3% (JP)* | EU, AUS |
| Uvinul A Plus | Diethylamino Hydroxybenzoyl Hexyl Benzoate | 10% (EU,JP) | EU, JP |
| Uvasorb HEB | Iscotrizinol, Diethylhexyl butamido triazone, DBT | 10% (EU) 5% (JP)* | EU, JP |
| Parsol SLX | Dimethico-diethylbenzalmalonate, Polysilicone-15 | 10% | EU, AUS, JP |
| Amiloxate | Isopentyl-4-methoxycinnamate, Isoamyl p-Methoxycinnamate, IMC, Neo Heliopan E1000 | 10%* | EU, AUS |
* Time and Extent Application (TEA), Proposed Rule on FDA approval originally expected 2009, now expected 2015.
Many of the ingredients awaiting approval by the FDA were relatively new, and developed to absorb UVA.[71] The 2014 Sunscreen Innovation Act was passed to accelerate the FDA approval process.[72][73]
Application
A sunscreen study from 2001 suggests that the best protection is achieved by dividing the SPF number in half and reapplying that many minutes after sun exposure begins. For example, if the SPF is 30, sunscreen should be reapplied once after 15 minutes of exposure. Further reapplication is only necessary after activities such as swimming, sweating, or rubbing/wiping.[74]
More-recent research at the University of California, Riverside, indicates that sunscreen must be reapplied within 2 hours in order to remain effective. Not reapplying could even cause more cell damage than not using sunscreen at all, due to the release of extra free radicals from those sunscreen chemicals that were absorbed into the skin.[62]
Dosage
The dose used in FDA sunscreen testing is 2 mg/cm2 of exposed skin.[52] If one assumes an "average" adult build of height 5 ft 4 in (163 cm) and weight 150 lb (68 kg) with a 32-inch (82-cm) waist, that adult wearing a bathing suit covering the groin area should apply approximately 30 g (or 30 ml, approximately 1 oz) evenly to the uncovered body area. This can be more easily thought of as a "golf ball" size amount of product per body, or at least six teaspoonfuls. Larger or smaller individuals should scale these quantities accordingly.[75] Considering only the face, this translates to about 1/4 to 1/3 of a teaspoon for the average adult face.
Some studies have shown that people commonly apply only 1/4 to 1/2 of the amount recommended for achieving the rated sun protection factor (SPF), and in consequence the effective SPF should be downgraded to a square root or 4th root of the advertised value.[46] A later study found a significant exponential relation between SPF and the amount of sunscreen applied, and the results are closer to linearity than expected by theory.[76]
FDA labeling regulations
Sunscreen labeling standards have been evolving in the United States since the FDA first adopted the SPF calculation in 1978.[77] The FDA issued a comprehensive set of rules in June 2011, taking effect in 2012–2013, designed to help consumers identify and select suitable sunscreen products offering protection from sunburn, early skin aging, and skin cancer:[41][78][79]
- To be classified as "broad spectrum", sunscreen products must provide protection against both UVA and UVB, with specific tests required for both.
- Claims of products being "waterproof" or "sweatproof" are prohibited, while "sunblock" and "instant protection" and "protection for more than 2 hours" are all prohibited without specific FDA approval.
- "Water resistance" claims on the front label must indicate how long the sunscreen remains effective and specify whether this applies to swimming or sweating, based on standard testing.
- Sunscreens must include standardized "Drug Facts" information on the container. However, there is no regulation that deems it necessary to mention whether the contents contain nanoparticles of mineral ingredients. (The EU has stricter regulation against the use of nanoparticles, and in 2009 introduced labeling requirements for nanoparticle ingredients in certain sunscreens and cosmetics.)[80]
Environmental effects
Certain sunscreens in water under ultraviolet light can increase the production of hydrogen peroxide, which damages phytoplankton.[81] Nanoparticles of titanium dioxide, an ingredient in some sunscreens, can accumulate in coastal waters and be ingested by marine animals.[81]
Notes
- ↑ "Preventing melanoma". Cancer Research UK. Retrieved 2009-09-22.
- ↑ Sunscreens | The Ageing Skin
- ↑ What You Need To Know About Skin Cancer
- ↑ Poon, Terence SC Poon; Barnetson, Ross StC; Halliday, Gary M (2003). "Prevention of Immunosuppression by Sunscreens in Humans Is Unrelated to Protection from Erythema and Dependent on Protection from Ultraviolet A in the Face of Constant Ultraviolet B Protection". J Invest Dermatol. 121: 184–90. doi:10.1046/j.1523-1747.2003.12317.x.
- 1 2 Hughes, MCB; Williams, GM; Baker, P; Green, AC (June 4, 2013). "Sunscreen and Prevention of Skin Aging". Annals of Internal Medicine. 158 (11): 781–790. doi:10.7326/0003-4819-158-11-201306040-00002.
- ↑ Kanavy HE, Gerstenblith MR (December 2011). "Ultraviolet radiation and melanoma". Semin Cutan Med Surg. 30 (4): 222–8. doi:10.1016/j.sder.2011.08.003. PMID 22123420.
- ↑ World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.14. ISBN 9283204298.
- ↑ Azoury, SC; Lange, JR (October 2014). "Epidemiology, risk factors, prevention, and early detection of melanoma.". The Surgical clinics of North America. 94 (5): 945–62, vii. doi:10.1016/j.suc.2014.07.013. PMID 25245960.
- ↑ Burnett M.E.; Wang S.Q. (April 2011). "Current sunscreen controversies: a critical review". Photodermatology, Photoimmunology & Photomedicine. 27 (2): 58–67. doi:10.1111/j.1600-0781.2011.00557.x. PMID 21392107.
- ↑ Kütting B, Drexler H (December 2010). "UV-induced skin cancer at workplace and evidence-based prevention". Int Arch Occup Environ Health. 83 (8): 843–54. doi:10.1007/s00420-010-0532-4. PMID 20414668.
- ↑ Dresbach S.H.; Brown W. (2008). "Ultraviolet Radiation" (PDF). Ohioline Fact Sheet Series. Ohio State University Extension.
- ↑ Australian Government: Therapeutic Goods Administration (July 2009). "A review of the scientific literature on the safety of nanoparticulate titanium dioxide or zinc oxide in sunscreens" (PDF). Archived from the original on April 6, 2011. Retrieved June 15, 2015.
- ↑ Norval, M; Wulf, HC (October 2009). "Does chronic sunscreen use reduce vitamin D production to insufficient levels?". The British journal of dermatology. 161 (4): 732–6. doi:10.1111/j.1365-2133.2009.09332.x. PMID 19663879.
- ↑ Holick MF (December 2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". Am. J. Clin. Nutr. 80 (6 Suppl): 1678S–1688S. PMID 15585788.
- ↑ Sayre, Robert M.; Dowdy, John C. (2007). "Darkness at Noon: Sunscreens and Vitamin D3". Photochemistry and Photobiology. 83 (2): 459–463. doi:10.1562/2006-06-29-RC-956. PMID 17115796.
- ↑ Holick MF (February 2002). "Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health". Current Opinion in Endocrinology, Diabetes and Obesity. 9 (1): 87–98. doi:10.1097/00060793-200202000-00011.
- ↑ Holick MF (September 2002). "Sunlight and Vitamin D". Journal of General Internal Medicine. 17 (9): 733–735. doi:10.1046/j.1525-1497.2002.20731.x. PMC 1495109
 . PMID 12220371.
. PMID 12220371. - ↑ Holick MF (July 2007). "Vitamin D deficiency". The New England Journal of Medicine. 357 (3): 266–281. doi:10.1056/NEJMra070553. PMID 17634462.
- 1 2 3 Shaath, Nadim A., editor (2005). Sunscreens: Regulations and Commercial Development, Third Edition. Taylor & Francis Group.
- ↑ Craddock, P.T. (1998). 2000 Years of Zinc and Brass. British Museum. p. 27. ISBN 0-86159-124-0.
- ↑ Craddock, P.T. (2008). "Mining and Metallurgy, chapter 4". In Oleson, John Peter. The Oxford Handbook of Engineering and Technology in the Classical World. Oxford University Press. pp. 111–112. ISBN 0-19-518731-8.
- 1 2 3 Rigel, Darrell S. (2004). Photoaging. Hoboken: Informa Healthcare. pp. 73–74. ISBN 9780824752095.
- ↑ Wang, Steven Q; Hu, Judy Y. "Challenges in Making an Effective Sunscreen". The Skin Cancer Foundation. Retrieved 2014-06-12.
- 1 2 3 Lim, Henry W. "Quantum Leaps: New, Improved Sunscreens Have Arrived". The Skin Cancer Foundation. Retrieved July 23, 2014.
- ↑ MacEachern, W.N.; Jillson, O.F. (January 1964). "A Practical Sunscreen — "Red Vet Pet"". Arch Dermatol. 89 (1): 147–150. doi:10.1001/archderm.1964.01590250153027. PMID 14070829. Retrieved July 24, 2014.
- ↑ "Sunscreen: A History". The New York Times. June 23, 2010. Retrieved July 24, 2014.
- ↑ "Gletscher Crème". 2010-04-22. Piz Buin. Archived from the original on 2010-05-12. Retrieved 2013-06-29.
- ↑ Lim, Henry W.; et al., eds. (2007). Photodermatology. CRC Press. p. 6. ISBN 9781420019964. Retrieved July 24, 2014.
- 1 2 Sunblock. UCSF. School of Medicine. Dept of Dermatology.
- ↑ "Sunscreen FAQs". American Academy of Dermatology. Retrieved July 22, 2014.
- ↑ Stege, H.; Budde; Grether-Beck; Richard; Rougier; Ruzicka; Krutmann (2002). "Sunscreens with high SPF values are not equivalent in protection from UVA induced polymorphous light eruption". European journal of dermatology : EJD. 12 (4): IV–VI. PMID 12118426.
- ↑ Haywood, R.; Wardman, P.; Sanders, R.; Linge, C. (2003). "Sunscreens inadequately protect against ultraviolet-A-induced free radicals in skin: implications for skin aging and melanoma?". The Journal of Investigative Dermatology. 121 (4): 862–868. doi:10.1046/j.1523-1747.2003.12498.x. PMID 14632206.
- ↑ Moyal, D.; Fourtanier, A. (2008). "Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings". Journal of the American Academy of Dermatology. 58 (5 Suppl 2): S149–S154. doi:10.1016/j.jaad.2007.04.035. PMID 18410801.
- ↑ Berneburg M, Plettenberg H, Medve-König K, Pfahlberg A, Gers-Barlag H, Gefeller O, Krutmann J (2004). "Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin". J Invest Dermatol. 122 (5): 1277–83. doi:10.1111/j.0022-202X.2004.22502.x. PMID 15140232.
- ↑ "Sunscreen makers sued for misleading claims". Associated Press. April 24, 2006. Retrieved January 5, 2015.
- ↑ Pinnell SR, Fairhurst D, Gillies R, Mitchnick MA, Kollias N (April 2000). "Microfine zinc oxide is a superior sunscreen ingredient to microfine titanium dioxide". Dermatol Surg. 26 (4): 309–14. doi:10.1046/j.1524-4725.2000.99237.x. PMID 10759815.
- ↑ "Commission Recommendation of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto". Official Journal of the European Union. 2006-09-22. Retrieved 2009-09-25.
- ↑ "UV Resource Guide - Sunscreens". Arpansa. 2008-12-20. Retrieved 2009-09-25.
- ↑ "SPF50+ Sunscreen". 2013-02-01. Retrieved 2014-02-06.
- 1 2 Questions and Answers on the 2007 Sunscreen Proposed Rule
- 1 2 3 4 "Questions and Answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the U.S.". 2011-06-23. Retrieved 2012-04-10.
- ↑ Department of Health and Human Services: Food and Drug Administration (June 17, 2011). "Revised Effectiveness Determination; Sunscreen Drug Products for Over-the-Counter Human Use" (PDF). Federal Register. 76 (117): 35672–35678. Retrieved November 21, 2013.
- 1 2 "Sunscreen Takes Some Heat: New Dangers, New Rules". 2011-06-16. Retrieved 2012-04-10.
- ↑ Moyal, Dominique (June 2008). "How to measure UVA protection afforded by suncreen products". Expert Rev. Dermatol. 3 (3): 307–313. doi:10.1586/17469872.3.3.307.
- ↑ "What to Know About Sunscreen Before Buying It". Consumer Reports. May 2014. Retrieved December 20, 2014.
- 1 2 Faurschou A, Wulf HC (April 2007). "The relation between sun protection factor and amount of sunscreen applied in vivo". Br. J. Dermatol. 156 (4): 716–9. doi:10.1111/j.1365-2133.2006.07684.x. PMID 17493070.
- ↑ Colipa UVA method
- ↑ www.cosmeticseurope.eu
- ↑ "Boots under fire as it bans low-price competitors from using its 'star rating' system for sun creams". The Daily Mail. July 1, 2011. Retrieved August 17, 2016.
- 1 2 "Nanotechnology Information Center: Properties, Applications, Research, and Safety Guidelines". American Elements.
- ↑ Schroeder P, Krutmann J (April 2010). "What is Needed for a Sunscreen to Provide Complete Protection". Skin Therapy Letter. 15 (4).
- 1 2 "Re: Tentative Final Monograph for OTC Sunscreen" (PDF). Food and Drug Administration (United States). 1998-09-11. Retrieved 2009-09-25.
- ↑ Neutrogena | How Helioplex Works
- ↑ Banana Boat AvoTriplex
- ↑ Chatelain E, Gabard B (September 2001). "Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter". Photochem Photobiol. 74 (3): 401–6. doi:10.1562/0031-8655(2001)074<0401:POBMAA>2.0.CO;2. PMID 11594052.
- ↑ "Parsol 340 – Octocrylene". DSM. Archived from the original on August 3, 2009. Retrieved June 22, 2015.
- ↑ Burke, Karen E. "Does sunscreen become ineffective with age?". The Skin Cancer Foundation. Retrieved July 31, 2014.
- ↑ Flindt-Hansen, H; Thune, P; Eeg-Larsen, T (1990). "The inhibiting effect of PABA on photocarcinogenesis". Archives of Dermatological Research. 282 (1): 38–41. doi:10.1007/BF00505643. PMID 2317082.
- ↑ Flindt-Hansen, H; Thune, P; Eeg-Larsen, T (1990). "The effect of short-term application of PABA on photocarcinogenesis". Acta Derm Venereol. 70 (1): 72–75. PMID 1967881.
- ↑ Osgood, PJ; Moss, SH; Davies, DJ (1982). "The sensitization of near-ultraviolet radiation killing of mammalian cells by the sunscreen agent para-aminobenzoic acid". Journal of Investigative Dermatology. 79 (6): 354–357. doi:10.1111/1523-1747.ep12529409. PMID 6982950.
- ↑ Mosley, C N; Wang, L; Gilley, S; Wang, S; Yu, H (2007). "Light-Induced Cytotoxicity and Genotoxicity of a Sunscreen Agent, 2-Phenylbenzimidazol in Salmonella typhimurium TA 102 and HaCaT Keratinocytes". International Journal of Environmental Research and Public Health. 4 (2): 126–131. doi:10.3390/ijerph2007040006. PMID 17617675.
- 1 2 Hanson, KM; Gratton, E; Bardeen, CJ (2006). "Sunscreen enhancement of UV-induced reactive oxygen species in the skin". Free Radical Biology and Medicine. 41 (8): 1205–12. doi:10.1016/j.freeradbiomed.2006.06.011. PMID 17015167.
- ↑ Nash, JF (2006). "Human Safety and Efficacy of Ultraviolet Filters and Sunscreen Products". Dermatologic Clinics. 24 (1): 35–51. doi:10.1016/j.det.2005.09.006. PMID 16311166.
- 1 2 Lautenschlager, Stephan; Wulf, Hans Christian; Pittelkow, Mark R (2007). "photoprotection". Lancet. 370 (9586): 528–37. doi:10.1016/S0140-6736(07)60638-2. PMID 17693182.
- ↑ Benech-Kieffer F, Meuling WJ, Leclerc C, Roza L, Leclaire J, Nohynek G (Nov–Dec 2003). "Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data". Skin Pharmacol Appl Skin Physiol. 16 (6): 343–55. doi:10.1159/000072929. PMID 14528058.
- ↑ Fourtanier A (October 1996). "Mexoryl SX protects against solar-simulated UVR-induced photocarcinogenesis in mice". Photochem Photobiol. 64 (4): 688–93. doi:10.1111/j.1751-1097.1996.tb03125.x. PMID 8863475.
- 1 2 "Regulation No. 1223/2009 on cosmetic products". Official Journal of the European Union. December 22, 2009. Retrieved May 26, 2015.
- ↑ "Opinion on Zinc oxide" (PDF). European Commission: Scientific Committee on Consumer Safety. April 22, 2014. Retrieved May 26, 2015.
- ↑ Australian Government: Therapeutic Goods Administration (November 2012). "Australian Regulatory Guidelines for Sunscreens". Retrieved June 21, 2015.
- ↑ "Uvinul Grades" (PDF). Retrieved 2009-09-25.
- ↑ Kapes, Beth (July 2005). "Docs rally for better sun protection — Advances still unavailable in United States". Dermatology Times. 26 (7): 100. Retrieved July 23, 2014.
- ↑ "Sunscreen Innovation Act". United States Congress. Retrieved January 5, 2015.
- ↑ Sifferlin, Alexandra (July 16, 2014). "We're One Step Closer to Better Sunscreen". Time. Retrieved August 1, 2014.
- ↑ Diffey B (2001). "When should sunscreen be reapplied?". J Am Acad Dermatol. 45 (6): 882–5. doi:10.1067/mjd.2001.117385. PMID 11712033.
- ↑ "How and why we use sunscreen". Cosmetic, Toiletry & Perfumery Association. Retrieved May 11, 2016.
- ↑ Schalka S, dos Reis VM, Cucé LC (August 2009). "The influence of the amount of sunscreen applied and its sun protection factor (SPF): evaluation of two sunscreens including the same ingredients at different concentrations". Photodermatol Photoimmunol Photomed. 25 (4): 175–80. doi:10.1111/j.1600-0781.2009.00408.x. PMID 19614894.
- ↑ Department of Health and Human Services: Food and Drug Administration (August 25, 1978). "Sunscreen Drug Products for Over-the-Counter Human Use" (PDF). Federal Register. 43 (166): 38206–38269. Retrieved July 30, 2014.
- ↑ Department of Health and Human Services: Food and Drug Administration (June 17, 2011). "Sunscreen Drug Products for Over-the-Counter Human Use; Final Rules and Proposed Rules" (PDF). Federal Register. 76 (117): 35620–35665. Retrieved August 19, 2014.
- ↑ Department of Health and Human Services: Food and Drug Administration (May 11, 2012). "Sunscreen Drug Products for Over-the-Counter Human Use; Delay of Compliance Dates" (PDF). Federal Register. 77 (92): 27591–27593. Retrieved September 27, 2012.
- ↑ "Is Sunscreen Safe?". Eluxe. June 8, 2014. Archived from the original on April 4, 2015.
- 1 2 Sánchez-Quiles D.; Tovar-Sánchez A. (2014). "Sunscreens as a source of hydrogen peroxide production in coastal waters". Environ Sci Technol. 48 (16): 9037–9042. doi:10.1021/es5020696. PMID 25069004.
External links
- FDA rulemaking history for sunscreens
- Environmental Working Group: July 2009 Sunscreen Safety Guide and Report
- Information on what sunscreens are and how they work from The Skin Cancer Foundation
- Sun Safety for Babies and Children University of Florida, IFAS Extension Department of Family, Youth and Community Sciences
- Article on UV absorbers not yet approved by the FDA
- Radiation protectants and their CAS registry number
- European Cosmetics ingredient database (CosIng)
- How does sunscreen work? Simple explanation from physics.org