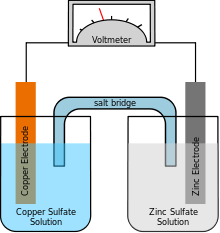
Rechargeable battery



A rechargeable battery, storage battery, secondary cell, or accumulator is a type of electrical battery which can be charged, discharged into a load, and recharged many times, while a non-rechargeable or primary battery is supplied fully charged, and discarded once discharged. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, nickel cadmium (NiCd), nickel metal hydride (NiMH), lithium ion (Li-ion), and lithium ion polymer (Li-ion polymer).
Rechargeable batteries typically initially cost more than disposable batteries, but have a much lower total cost of ownership and environmental impact, as they can be recharged inexpensively many times before they need replacing. Some rechargeable battery types are available in the same sizes and voltages as disposable types, and can be used interchangeably with them.
Usage and applications

Devices which use rechargeable batteries include automobile starters, portable consumer devices, light vehicles (such as motorized wheelchairs, golf carts, electric bicycles, and electric forklifts), tools, uninterruptible power supplies, and battery storage power stations. Emerging applications in hybrid internal combustion-battery and electric vehicles drive the technology to reduce cost, weight, and size, and increase lifetime.[1]
Older rechargeable batteries self-discharge relatively rapidly, and require charging before first use; some newer low self-discharge NiMH batteries hold their charge for many months, and are typically sold factory-charged to about 70% of their rated capacity.
Battery storage power stations use rechargeable batteries for load-leveling (storing electric energy at times of low demand for use during peak periods) and for renewable energy uses (such as storing power generated from photovoltaic arrays during the day to be used at night). Load-leveling reduces the maximum power which a plant must be able to generate, reducing capital cost and the need for peaking power plants.
The US National Electrical Manufacturers Association estimated in 2006 that US demand for rechargeable batteries was growing twice as fast as demand for disposables.[2]
Small rechargeable batteries can power portable electronic devices, power tools, appliances, and so on. Heavy-duty batteries power electric vehicles, ranging from scooters to locomotives and ships. They are used in distributed electricity generation and in stand-alone power systems.
Charging and discharging

During charging, the positive active material is oxidized, producing electrons, and the negative material is reduced, consuming electrons. These electrons constitute the current flow in the external circuit. The electrolyte may serve as a simple buffer for internal ion flow between the electrodes, as in lithium-ion and nickel-cadmium cells, or it may be an active participant in the electrochemical reaction, as in lead–acid cells.
The energy used to charge rechargeable batteries usually comes from a battery charger using AC mains electricity, although some are equipped to use a vehicle's 12-volt DC power outlet. Regardless, to store energy in a secondary cell, it has to be connected to a DC voltage source. The negative terminal of the cell has to be connected to the negative terminal of the voltage source and the positive terminal of the voltage source with the positive terminal of the battery. Further, the voltage output of the source must be higher than that of the battery, but not much higher: the greater the difference between the power source and the battery's voltage capacity, the faster the charging process, but also the greater the risk of overcharging and damaging the battery.
Chargers take from a few minutes to several hours to charge a battery. Slow "dumb" chargers without voltage or temperature-sensing capabilities will charge at a low rate, typically taking 14 hours or more to reach a full charge. Rapid chargers can typically charge cells in two to five hours, depending on the model, with the fastest taking as little as fifteen minutes. Fast chargers must have multiple ways of detecting when a cell reaches full charge (change in terminal voltage, temperature, etc.) to stop charging before harmful overcharging or overheating occurs. The fastest chargers often incorporate cooling fans to keep the cells from overheating.

Battery charging and discharging rates are often discussed by referencing a "C" rate of current. The C rate is that which would theoretically fully charge or discharge the battery in one hour. For example, trickle charging might be performed at C/20 (or a "20 hour" rate), while typical charging and discharging may occur at C/2 (two hours for full capacity). The available capacity of electrochemical cells varies depending on the discharge rate. Some energy is lost in the internal resistance of cell components (plates, electrolyte, interconnections), and the rate of discharge is limited by the speed at which chemicals in the cell can move about. For lead-acid cells, the relationship between time and discharge rate is described by Peukert's law; a lead-acid cell that can no longer sustain a usable terminal voltage at a high current may still have usable capacity, if discharged at a much lower rate. Data sheets for rechargeable cells often list the discharge capacity on 8-hour or 20-hour or other stated time; cells for uninterruptible power supply systems may be rated at 15 minute discharge.
Battery manufacturers' technical notes often refer to voltage per cell (VPC) for the individual cells that make up the battery. For example, to charge a 12 V lead-acid battery (containing 6 cells of 2 V each) at 2.3 VPC requires a voltage of 13.8 V across the battery's terminals.
Non-rechargeable alkaline and zinc–carbon cells output 1.5V when new, but this voltage drops with use. Most NiMH AA and AAA cells are rated at 1.2 V, but have a flatter discharge curve than alkalines and can usually be used in equipment designed to use alkaline batteries.
Damage from cell reversal
Subjecting a discharged cell to a current in the direction which tends to discharge it further to the point the positive and negative terminals switch polarity causes a condition called cell reversal. Generally, pushing current through a discharged cell in this way causes undesirable and irreversible chemical reactions to occur, resulting in permanent damage to the cell. Cell reversal can occur under a number of circumstances, the two most common being:
- When a battery or cell is connected to a charging circuit the wrong way around.
- When a battery made of several cells connected in series is deeply discharged.
In the latter case, the problem occurs due to the different cells in a battery having slightly different capacities. When one cell reaches discharge level ahead of the rest, the remaining cells will force the current through the discharged cell.
Many battery-operated devices have a low-voltage cutoff that prevents deep discharges from occurring that might cause cell reversal.
Cell reversal can occur to a weakly charged cell even before it is fully discharged. If the battery drain current is high enough, the cell's internal resistance can create a resistive voltage drop that is greater than the cell's forward emf. This results in the reversal of the cell's polarity while the current is flowing.[3][4] The higher the required discharge rate of a battery, the better matched the cells should be, both in the type of cell and state of charge, in order to reduce the chances of cell reversal.
In some situations, such as when correcting Ni-Cad batteries that have been previously overcharged,[5] it may be desirable to fully discharge a battery. To avoid damage from the cell reversal effect, it is necessary to access each cell separately: each cell is individually discharged by connecting a load clip across the terminals of each cell, thereby avoiding cell reversal.
Damage during storage in fully discharged state
If a multi-cell battery is fully discharged, it will often be damaged due to the cell reversal effect mentioned above. It is possible however to fully discharge a battery without causing cell reversal—either by discharging each cell separately, or by allowing each cell's internal leakage to dissipate its charge over time.
Even if a cell is brought to a fully discharged state without reversal, however, damage may occur over time simply due to remaining in the discharged state. An example of this is the sulfation that occurs in lead-acid batteries that are left sitting on a shelf for long periods. For this reason it is often recommended to charge a battery that is intended to remain in storage, and to maintain its charge level by periodically recharging it. Since damage may also occur if the battery is overcharged, the optimal level of charge during storage is typically around 30% to 70%.
Depth of discharge
Depth of discharge (DOD) is normally stated as a percentage of the nominal ampere-hour capacity; 0% DOD means no discharge. As the usable capacity of a battery system depends on the rate of discharge and the allowable voltage at the end of discharge, the depth of discharge must be qualified to show the way it is to be measured. Due to variations during manufacture and aging, the DOD for complete discharge can change over time or number of charge cycles. Generally a rechargeable battery system will tolerate more charge/discharge cycles if the DOD is lower on each cycle.[6]
Lifespan and cycle stability
If batteries are used repeatedly even without mistreatment, they lose capacity as the number of charge cycles increases, until they are eventually considered to have reached the end of their useful life.
Lithium iron phosphate batteries reach according to the manufacturer more than 5000 cycles at respective depth of discharge of 70%.[7] After 7500 cycles with discharge of 85% this still have a spare capacity of at least 80% at a rate of 1 C; which corresponds with a full cycle per day to a lifetime of min. 20.5 years.
The lithium iron phosphate battery Sony Fortelion has after 10,000 cycles at 100% discharge level still a residual capacity of 71%. This battery has been on the market since 2009.[8]
Used in solar batteries Lithium-ion batteries have partly a very high cycle resistance of more than 10,000 charge and discharge cycles and a long service life of up to 20 years.[9][10]
Plug in America has among drivers of the Tesla Roadster, a survey carried out with respect to the service life of the installed battery. It was found that after 100,000 miles = 160,000 km, the battery still had a remaining capacity of 80 to 85 percent. This was regardless of in which climate zone the car is moved.[11][12] The Tesla Roadster was built and sold between 2008 and 2012. For its 85-kWh batteries in the Tesla Model S Tesla are 8-year warranty with unlimited mileage.[13]
Varta Storage guarantees its engion battery systems for 14,000 full cycles and a service life of 10 years.[14][15]
The best-selling electric car is the Nissan Leaf, which is produced since of 2010. Nissan stated in 2015 that until then only 0.01 percent of batteries had to be replaced because of failures or problems and then only because of externally inflicted damage. There are few vehicles that have already covered more than 200,000 km away. These have no problems with the battery.[16]
Recharging time

Electric cars like Tesla Model S, Renault Zoe, BMW i3, etc. can recharge their batteries at quick charging stations within 30 minutes to 80 percent.[17][18][19][20]
In laboratories the company StoreDot from Israel reportedly demonstrated the first lab samples of unspecified batteries that can, as of April 2014, be charged in 30 seconds in mobile phones.[21][22]
Researchers from Singapore in 2014 developed a battery that can be recharged in 2 minutes to 70 percent. The batteries rely on lithium-ion technology. However, the anode and the negative pole in the battery is no longer made of graphite, but a titanium dioxide gel. The gel accelerates the chemical reaction significantly, thus ensuring a faster charging. In particular, these batteries are to be used in electric cars.[23][24][25] Already in 2012 researchers at the Ludwig-Maximilian-University in Munich have discovered the basic principle.[26]
Scientists at Stanford University in California have developed a battery that can be charged within one minute. The anode is made of aluminum and the cathode made of graphite (see Aluminium-ion battery).[27][28]
The electric car Volar-e of the company Applus + IDIADA, based on the Rimac Concept One, contains lithium iron phosphate batteries that can be recharged in 15 minutes.[29]
According to the manufacturer BYD the lithium iron phosphate battery of the electric car e6 is charged at a fast charging station within 15 minutes to 80%, after 40 minutes at 100%.[30]
In 2005, handheld device battery designs by Toshiba were claimed to be able to accept an 80% charge in as little as 60 seconds.[31]
Scientists of university of Oslo from Norway have developed a battery which can be recharged less than one second. According to the scientists this battery would be interesting for example for city buses, which could be loaded at each bus stop, and thus would require only a relatively small battery. A disadvantage is, according to the researchers that the bigger the battery, the greater must be the charging current. Thus, the battery can not be very big. According to the researchers of the new battery could also be used as a buffer in sports car to provide power in the short term. For now, however, the researchers think of applications in small and micro devices.[32][33]
According to the manufacturer battery of the smartphone OnePlus 3 can be charged from 0 to 60 percent within 30 minutes.[34]
Price history
Lead-acid batteries typically cost €100 / kWh. Li-Ion batteries cost in January 2014, however, typically around €110 / kWh (150 USD / kWh). The prices for Li-Ion batteries are since 2011 dropped significantly (2011: €500 / kWh, 2012: €350 / kWh, 2013: €200 / kWh) [35][36][37][38][39] At a conference for electric mobility October 2013 mentioned the trend researcher Lars Thomsen, that Tesla has built its battery at the time 200 USD / kWh (equivalent to €148 / kWh).[40] for the planned for autumn 2016 e-mobile Bolt expects General Motors 145 USD / kWh, and a reduction to 100 USD / kWh by 2022.[41] Reasons for the price decline is the increasing mass production, which has reduced costs through better technologies and economies of scale.
In the German retail LiFePO4 battery cells (as of January 2015) are available from about 420 € / kWh (1.35 € / Ah).[42]
The Powerpack of Tesla costs in spring 2016 250 USD per kWh [43] (see Tesla Powerwall).
Active components
The active components in a secondary cell are the chemicals that make up the positive and negative active materials, and the electrolyte. The positive and negative are made up of different materials, with the positive exhibiting a reduction potential and the negative having an oxidation potential. The sum of these potentials is the standard cell potential or voltage.
In primary cells the positive and negative electrodes are known as the cathode and anode, respectively. Although this convention is sometimes carried through to rechargeable systems—especially with lithium-ion cells, because of their origins in primary lithium cells—this practice can lead to confusion. In rechargeable cells the positive electrode is the cathode on discharge and the anode on charge, and vice versa for the negative electrode.
Types
The lead–acid battery, invented in 1859 by French physicist Gaston Planté, is the oldest type of rechargeable battery. Despite having a very low energy-to-weight ratio and a low energy-to-volume ratio, its ability to supply high surge currents means that the cells have a relatively large power-to-weight ratio. These features, along with the low cost, makes it attractive for use in motor vehicles to provide the high current required by automobile starter motors.
The nickel–cadmium battery (NiCd) was invented by Waldemar Jungner of Sweden in 1899. It uses nickel oxide hydroxide and metallic cadmium as electrodes. Cadmium is a toxic element, and was banned for most uses by the European Union in 2004. Nickel–cadmium batteries have been almost completely superseded by nickel–metal hydride (NiMH) batteries.
The nickel–metal hydride battery (NiMH) became available in 1989.[44] These are now a common consumer and industrial type. The battery has a hydrogen-absorbing alloy for the negative electrode instead of cadmium.
The lithium-ion battery was introduced in the market in 1991, and it is the choice in most consumer electronics and has the best energy density and a very slow loss of charge when not in use.
Lithium-ion polymer batteries are light in weight, offer slightly higher energy density than Li-ion at slightly higher cost, and can be made in any shape. They are available[45] but have not displaced Li-ion in the market.
Experimental types
| Type | Voltagea | Energy densityb | Powerc | E/$e | Self-disch.f | Charge Efficiency | Cyclesg | Lifeh | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (V) | (MJ/kg) | (Wh/kg) | (Wh/L) | (W/kg) | (Wh/$) | (%/month) | (%) | (#) | (years) | |
| Lithium sulfur[46] | 2.0 | 0.94-1.44[47] | 400[48] | 350 | ~1400[49] | |||||
| Sodium-ion[50] | 3.6 | 30 | 3.3 | 5000+ | Testing | |||||
| Thin film lithium | ? | 300[51] | 959[51] | 6000[51] | ?p[51] | 40000[51] | ||||
| Zinc-bromide | 0.27-0.31 | 75-85 | ||||||||
| Zinc-cerium | 2.5[52] | Under testing | ||||||||
| Vanadium redox | 1.15-1.55 | 0.09-0.13 | 25-35[53] | 20%[54] | 14,000[55] | 10 (stationary)[54] | ||||
| Sodium-sulfur | 0.54 | 150 | 89–92% | 2500—4500 | ||||||
| Molten salt | 2.58 | 0.25-1.04 | 70-290[56] | 160[57] | 150-220 | 4.54[58] | 3000+ | <=20 | ||
| Silver-zinc | 1.86 | 0.47 | 130 | 240 | ||||||
| Quantum Battery (oxide semiconductor)[59][60] | 1.5-3 | 500 | 8000(W/L) | 100,000 | ||||||
‡ citations are needed for these parameters
- Notes
- a Nominal cell voltage in V.
- b Energy density = energy/weight or energy/size, given in three different units
- c Specific power = power/weight in W/kg
- e Energy/consumer price in W·h/US$ (approximately)
- f Self-discharge rate in %/month
- g Cycle durability in number of cycles
- h Time durability in years
- i VRLA or recombinant includes gel batteries and absorbed glass mats
- p Pilot production
The lithium–sulfur battery was developed by Sion Power in 1994.[61] The company claims superior energy density to other lithium technologies.[62]
The thin film battery (TFB) is a refinement of lithium ion technology by Excellatron.[63] The developers claim a large increase in recharge cycles to around 40,000 and higher charge and discharge rates, at least 5 C charge rate. Sustained 60 C discharge and 1000C peak discharge rate and a significant increase in specific energy, and energy density.[64]
A smart battery has voltage monitoring circuit built inside. Carbon foam-based lead acid battery: Firefly Energy developed a carbon foam-based lead acid battery with a reported energy density of 30-40% more than their original 38 Wh/kg,[65] with long life and very high power density.
UltraBattery, a hybrid lead-acid battery and ultracapacitor invented by Australia’s national science organisation CSIRO, exhibits tens of thousands of partial state of charge cycles and has outperformed traditional lead-acid, lithium and NiMH-based cells when compared in testing in this mode against variability management power profiles.[66] UltraBattery has kW and MW-scale installations in place in Australia, Japan and the U.S.A. It has also been subjected to extensive testing in hybrid electric vehicles and has been shown to last more than 100,000 vehicle miles in on-road commercial testing in a courier vehicle. The technology is claimed to have a lifetime of 7 to 10 times that of conventional lead-acid batteries in high rate partial state-of-charge use, with safety and environmental benefits claimed over competitors like lithium-ion. Its manufacturer suggests an almost 100% recycling rate is already in place for the product.
The potassium-ion battery delivers around a million cycles, due to the extraordinary electrochemical stability of potassium insertion/extraction materials such as Prussian blue.
The sodium-ion battery is meant for stationary storage and competes with lead–acid batteries. It aims at a low total cost of ownership per kWh of storage. This is achieved by a long and stable lifetime. The effective number of cycles is above 5000 and the battery is not damaged by deep discharge. The energy density is rather low, somewhat lower than lead–acid.
The quantum battery (oxide semiconductor) was developed by MJC. It is a small, lightweight cell with a multi-layer film structure and high energy and high power density. It is incombustible, has no electrolyte and generates a low amount of heat during charge. Its unique feature is its ability to capture electrons physically rather than chemically.[67]
In 2007, Yi Cui and colleagues at Stanford University's Department of Materials Science and Engineering discovered that using silicon nanowires as the anode of a lithium-ion battery increases the anode's volumetric charge density by up to a factor of 10, leading to the development of the nanowire battery.[68]
Another development is the paper-thin flexible self-rechargeable battery combining a thin-film organic solar cell with an extremely thin and highly flexible lithium-polymer battery, which recharges itself when exposed to light.[69]
Ceramatec, a research and development unit of CoorsTek, as of 2009 was testing a battery comprising a chunk of solid sodium metal mated to a sulfur compound by a paper-thin ceramic membrane which conducts ions back and forth to generate a current. The company claimed that it could fit about 40 kilowatt hours of energy into a package about the size of a refrigerator, and operate below 90 °C; and that their battery would allow about 3,650 discharge/recharge cycles (or roughly 1 per day for one decade).[70]
Battery electrodes can be microscopically viewed while bathed in wet electrolytes, resembling conditions inside operating batteries.[71]
In 2014, an Israeli company, StoreDot, claimed to be able to charge batteries in 30 seconds.[72][73][74]
Secondary magnesium battery types are an active (2015) topic of research, as a replacement for lithium ion cells.
Aluminium-ion battery types had big success in 2015 in research.
Alternatives
A rechargeable battery is only one of several types of rechargeable energy storage systems.[75] Several alternatives to rechargeable batteries exist or are under development. For uses such as portable radios, rechargeable batteries may be replaced by clockwork mechanisms which are wound up by hand, driving dynamos, although this system may be used to charge a battery rather than to operate the radio directly. Flashlights may be driven by a dynamo directly. For transportation, uninterruptible power supply systems and laboratories, flywheel energy storage systems store energy in a spinning rotor for conversion to electric power when needed; such systems may be used to provide large pulses of power that would otherwise be objectionable on a common electrical grid.
Ultracapacitors—capacitors of extremely high value— are also used; an electric screwdriver which charges in 90 seconds and will drive about half as many screws as a device using a rechargeable battery was introduced in 2007,[76] and similar flashlights have been produced. In keeping with the concept of ultracapacitors, betavoltaic batteries may be utilized as a method of providing a trickle-charge to a secondary battery, greatly extending the life and energy capacity of the battery system being employed; this type of arrangement is often referred to as a "hybrid betavoltaic power source" by those in the industry.[77]
Ultracapacitors are being developed for transportation, using a large capacitor to store energy instead of the rechargeable battery banks used in hybrid vehicles. One drawback of capacitors compared to batteries is that the terminal voltage drops rapidly; a capacitor that has 25% of its initial energy left in it will have one-half of its initial voltage. By contrast, battery systems tend to have a terminal voltage that does not decline rapidly until nearly exhausted. The undesirable characteristic complicates the design of power electronics for use with ultracapacitors. However, there are potential benefits in cycle efficiency, lifetime, and weight compared with rechargeable systems. China started using ultracapacitors on two commercial bus routes in 2006; one of them is route 11 in Shanghai.[78]
Flow batteries, used for specialized applications, are recharged by replacing the electrolyte liquid. A flow battery can be considered to be a type of rechargeable fuel cell.
Research
Lithium Ion batteries normally have an anode made of graphite. Using an anode made of silicon (Si) can increase the capacity up to 6 times, because the Si-anode can accept much more Lithium-ion than a graphite-anode. A problem was, that the Si-anode expands 300–400% when charged. The Si-anode had only a small lifespan. Researchers of the university of Stuttgart (institute of photovoltaic (IPV), Prof. Dr. Jürgen H. Werner and his team) found a way making the Si-anode porous, so that accepting so many Lithium-ion will not longer increase the volume of the Si-anode, so that the lifespan of the battery with Si-anode is now four times higher than batteries with graphite-anode. The battery is ready for production.[79][80]
See also
References
- ↑ David Linden, Thomas B. Reddy (ed). Handbook Of Batteries 3rd Edition. McGraw-Hill, New York, 2002 ISBN 0-07-135978-8 chapter 22.
- ↑ "Batteries Product Stewardship | Wastes | EPA". Epa.gov. 2006-06-28. Retrieved 2012-08-14.
- ↑ Sequeira, C.A.C. Solid state batteries, North Atlantic Treaty Organization, Scientific Affairs Division, pp. 242-247, 254-259
- ↑ AEROSPACE CORP EL SEGUNDO CA CHEMISTRY AND PHYSICS LAB. Nickel-Cadmium Battery Cell Reversal from Resistive Network Effects: Computer simulations of short-down on a variety of battery configurations, DTIC Online website.
- ↑ Zaun, James A. NiCd Batteries do NOT have "memory", RepairFAQ.org website, 24 September 1996.
- ↑ Reddy, Handbook of Batteries page 22-20
- ↑ 3xe-electric-cars.com: Winston Battery, Herstellerangaben, abgerufen am 31. März 2014
- ↑ "Archived copy" (PDF). Archived from the original on 6 June 2014. Retrieved 2016-02-10., PDF, eingefügt am 3. Juni 2014.
- ↑ "Archived copy". Archived from the original on 24 June 2014. Retrieved 2016-02-10. "Die Tests setzten die Batterien extremen Belastungen aus. So wurden über einen Zeitraum von 5 Jahren bei einer Entladungstiefe von 60 % mehr als 10.000 äquivalente Vollzyklen erreicht." und „Simulationen, die sich auf unsere Laborergebnisse und die unserer Kollegen vom ZSW stützen, zeigen, dass bei Berücksichtigung beider Alterungsprozesse die Batterien im BPT-S 5 Hybrid bis zu 20 Jahre betriebsfähig sind“, abgerufen am 29. März 2014.
- ↑ "Archived copy". Archived from the original on 15 July 2014. Retrieved 2016-02-10., solarserver.de, abgerufen am 29. März 2014.
- ↑ Tesla Roadster – Batterie langlebiger als erwartet, greenmotorsblog.de, abgerufen am 31. März 2014
- ↑ Plug In America Research Shows That Tesla Roadster Battery Performance Bests Tesla Motors’ Own Projections, pluginamerica.org, abgerufen am 31. März 2014
- ↑ „Batteriegarantie: 8 Jahre, unbegrenzte km“, teslamotors.com, abgerufen am 5. April 2014.
- ↑ „VARTA Storage garantiert 14.000 Zyklen bei Batteriespeichern“, vom 13. Juli 2015, abgerufen am 13. Juli 2015.
- ↑ „VARTA Storage erweitert Garantie für Batteriespeicher auf 14.000 Zyklen“, abgerufen am 13. Juli 2015.
- ↑ „zeit.de: Batterie-Upgrade? Unwahrscheinlich!“, abgerufen am 22. Februar 2016.
- ↑ BMU, März 2011: Neue Stromtankstelle: Elektroautos laden in 20 Minuten, golem.de
- ↑ Die Ladezeit dauert je nach Station zwischen 30 Minuten (Gleichstrom-Ladestation) und etwa acht Stunden (Haushaltssteckdose), zeit.de
- ↑ Die Akkus im Renault Zoe können in der schnellsten von vier Ladegeschwindigkeiten in 30 Minuten bis zu 80 Prozent aufgeladen werden, bild.de
- ↑ Mit einem Schnellladegerät lässt sich der Akku des i3 in nur 30 Minuten zu 80 Prozent aufladen, golem.de
- ↑ Galaxy S4 in 30 Sekunden geladen: StoreDot demonstriert neue Akkutechnik, netzwelt.de, aufgerufen 9. April 2014
- ↑ 'Günstiger' Quantenpunkt-Akku lädt in 30 Sekunden, winfuture.de, aufgerufen 9. April 2014
- ↑ "Archived copy". Archived from the original on 4 February 2015. Retrieved 2016-02-10., bluewin.ch, aufgerufen 30. Dezember 2014
- ↑ „Ultra-fast charging batteries that can be 70% recharged in just two minutes“, sciencedaily.com, aufgerufen 30. Dezember 2014
- ↑ „Neuer Akku lädt in wenigen Minuten“, golem.de, aufgerufen 30. Dezember 2014
- ↑ „Lithium-Titan lädt in Sekunden“, elektroniknet.de, aufgerufen 30. Dezember 2014
- ↑ „Forscher: Aluminium-Speicher ‚hat alles, was man sich für eine Batterie erträumen kann‘“, ecomento.tv, aufgerufen 14. Mai 2015
- ↑ „An ultrafast rechargeable aluminium-ion battery“, nature.com, aufgerufen 14. Mai 2015
- ↑ "Elektroauto mit 1088 PS entwickelt" (in German). 2014-10-24. Retrieved 2015-04-12.
- ↑ byd-auto.net Website of BYD: 40(min) / 15(min 80%)
- ↑ Toshiba Corporation (2005) "Toshiba's New Rechargeable Lithium-Ion Battery Recharges in Only One Minute" press release at toshiba.co.jp. Retrieved 5 July 2006.
- ↑ cleantechnica.com Ridiculously-Fast-Charging Batteries (Not Supercapacitors) Developed In Norway
- ↑ tu.no De har lagt grunnlaget for batterier som kan lades 1000 ganger raskere
- ↑ phonearena.com OnePlus 3's Dash charging solution is fast and cool: 63% of battery juice in 30 minutes, 14. June 2016
- ↑ M. Seiwert, R. Böhmer, J. Rees, F. W. Rother: E-Auto-Batterien: Daimler und Evonik suchen Partner für Li-Tec. Online auf Wiwo.de vom 15.Juni 2013. Darin Audi-Chef Rupert Stadler: „Vor drei Jahren lagen die Preise pro Kilowattstunde noch bei 500 Euro … jetzt sind es rund 200 Euro. Und ich gehe davon aus, dass das nicht das Ende ist.“
- ↑ Batterien für Elektroautos werden immer günstiger, Elektroauto, Februar 2012, aufgerufen 19. Mai 2012
- ↑ Kosten für Batterien deutlich gesunken, t-online.de, aufgerufen 5. Dezember 2013
- ↑ „Tesla kauft seine Zellen von Panasonic/Sanyo für vermutlich 150 Dollar/Kilowattstunde“, schätzt Prof. Dirk Uwe Sauer von der RWTH, cleanthinking.de, aufgerufen 28. Januar 2014
- ↑ „I’m finding Chevy Volt replacement batteries online for about $2,300. $2,300/16 kWh = $144/kWh, Retail.“, cleantechnica.com, aufgerufen 18. März 2014
- ↑ Trendforscher erwartet baldigen Durchbruch der E-Autos zeit.de, mit Video des Vortrags. Zur Zeitangabe „Mitte 2014“ für 120 USD/kWh siehe Video des Vortrags.
- ↑ Chevrolet Bolt battery cells to cost "industry-leading" $145 per kWh,GM: Chevrolet Bolt Arrives In 2016, $145/kWh Cell Cost, Volt Margin Improves $3,500
- ↑ http://shop.lipopower.de/Winston_1 abgerufen am 2. Januar 2015
- ↑ "Tesla Energy: $250/kWh price point reached". transportevolved.com. Retrieved 2016-03-20.
- ↑ Katerina E. Aifantis et al, High Energy Density Lithium Batteries: Materials, Engineering, Applications Wiley-VCH, 2010 ISBN 3-527-32407-0 page 66
- ↑ all-battery.com: Lithium Polymer Batteries
- ↑ Lithium_Sulfur Archived 14 December 2007 at the Wayback Machine.
- ↑ "Solar plane makes record flight". BBC News. 24 August 2008. Retrieved 10 April 2010.
- ↑ Patent 6358643, PolyPlus.com website. Archived 18 March 2009 at the Wayback Machine.
- ↑ Research News: A longer life for lithium-sulfur batteries, Fraunhofer.de website, April 2013.
- ↑ Bullis, Kevin (2014-02-18). "How to Make a Cheap Battery for Storing Solar Power | MIT Technology Review". Technologyreview.com. Retrieved 2014-04-27.
- 1 2 3 4 5 "the Company". Excellatron. Retrieved 2012-08-14.
- ↑ Xie, Z.; Liu, Q.; Chang, Z.; Zhang, X. (2013). "The developments and challenges of cerium half-cell in zinc–cerium redox flow battery for energy storage". Electrochimica Acta. 90: 695–704. doi:10.1016/j.electacta.2012.12.066.
- ↑ "Vanadium Redox Battery". Vrb.unsw.edu.au. Retrieved 2012-08-14.
- 1 2 broken link
- ↑ The Vanadium Advantage: Flow Batteries Put Wind Energy in the Bank Archived 7 September 2008 at the Wayback Machine.
- ↑ "Sumitomo considering marketing new lower-temperature molten-salt electrolyte battery to automakers for EVs and hybrids". Green Car Congress. 2011-11-11. Retrieved 2012-04-24.
- ↑ "mpoweruk.com: Accumulator and battery comparisons (pdf)" (PDF). Retrieved 2012-08-14.
- ↑ "EVWORLD FEATURE: Fuel Cell Disruptor - Part 2:BROOKS FUEL CELL | CARB | ARB | HYDROGEN | ZEBRA | EV | ELECTRIC". Evworld.com. Archived from the original on 25 May 2012. Retrieved 2012-08-14.
- ↑ "Study of secondary battery semiconductor" (PDF). Hiroshima University. 2011-11-25. Retrieved 2014-01-18.
- ↑ "Notice of the development of mass production technology of Secondary battery "battenice" based on quantum technology" (PDF). MICRONICS JAPAN. 2013-11-19. Retrieved 2014-01-18.
- ↑ "Sion Power Corporation - Advanced Energy Storage : Welcome". Sionpower.com. Retrieved 2012-08-14.
- ↑ "Sion Power Corporation - Advanced Energy Storage : Technology Overview". Sionpower.com. Retrieved 2012-08-14.
- ↑ "Excellatron". Excellatron. 2010-06-02. Retrieved 2012-08-14.
- ↑ "the Company". Excellatron. Retrieved 2012-08-14.
- ↑ "Firefly Energy Eyeing the Hybrid Market; Lead-Acid Foam Batteries for Mild-Hybrid Applications Heading to DOE for Testing and Validation". Green Car Congress. 2008-01-12. Retrieved 2012-08-14.
- ↑ "Life Cycle Testing and Evaluation of Energy Storage Devices" (PDF). 2 January 2011. Retrieved 26 December 2014.
- ↑ "World Smart Energy Week 2014 e-Guidebook". Reed Exhibitions Japan Ltd. Retrieved 2014-01-18.
- ↑ Serpo, Alex (15 January 2008). "A tenfold improvement in battery life?". News.com. CNET. Retrieved 2008-04-12.
"High-performance lithium battery anodes using silicon nanowires". Nanotechnology 3, 31 - 35 (2008). Nature. 16 December 2007. doi:10.1038/nnano.2007.411. Retrieved 2008-04-12. - ↑ "Technology Review, Flexible Batteries That Never Need to Be Recharged, 2007". Technologyreview.com. 2007-04-04. Retrieved 2012-08-14.
- ↑ "New battery could change the world, one house at a time". Heraldextra.com. 2009-04-04. Retrieved 2012-08-14.
- ↑ Moving Forward With Rechargeable Battery Research, EET India, 30 December 2013
- ↑ Lilien, Niv (2014-04-09). "StoreDot: Inside the nanotech that can charge your phone in 30 seconds". ZDNet. Retrieved 2014-04-24.
- ↑ "Samsung spots startup's quantum-dot potential". Power-eetimes.com. 2013-11-14. Retrieved 2014-04-24.
- ↑ Jared Newman (2014-04-07). "StoreDot Phone Charging Works in 30 Seconds, but There's a Catch". TIME. Retrieved 2014-04-24.
- ↑ Miller, Charles. Illustrated Guide to the National Electrical Code, p. 445 (Cengage Learning 2011).
- ↑ "Capacitor-powered electric screwdriver, 2007". Ohgizmo.com. 2005-07-24. Retrieved 2012-08-14.
- ↑ Welcome to City Labs, CityLabs.net website.
- ↑ 超级电容公交车专题 (Super capacitor buses topics), 52Bus.com website, August 2006 (in Chinese, archived page).
- ↑ The potential of silicon anode based lithium ion batteries, sciencedirect.com, July 2016.
- ↑ Größere Reichweite und längere Lebensdauer für E-Fahrzeuge, oekonews.at, September 2016.
Further reading
- Belli, Brita. ‘Battery University’ Aims to Train a Work Force for Next-Generation Energy Storage, The New York Times, 8 April 2013. Discusses a professional development program at San Jose State University.
- Vlasic, Bill. Chinese Firm Wins Bid for Auto Battery Maker, The New York Times, published online 9 December 2012, p. B1.
- Cardwell, Diane. Battery Seen as Way to Cut Heat-Related Power Losses, 16 July 2013 online and 17 July 2013 in print on 17 July 2013, on page B1 in the New York City edition of The New York Times, p. B1. Discusses Eos Energy Systems' Zinc–air batteries.
- Cardwell, Diane. SolarCity to Use Batteries From Tesla for Energy Storage, 4 December 2013 on line, and 5 December 2013 in the New York City edition of The New York Times, p. B-2. Discusses SolarCity, DemandLogic and Tesla Motors.
- Galbraith, Kate. In Presidio, a Grasp at the Holy Grail of Energy Storage, The New York Times, 6 November 2010.
- Galbraith, Kate. Filling the Gaps in the Flow of Renewable Energy, The New York Times, 22 October 2013.
- Witkin, Jim. Building Better Batteries for Electric Cars, The New York Times, 31 March 2011, p. F4. Published online 30 March 2011. Discusses rechargeable batteries and the new-technology lithium ion battery.
- Wald, Matthew L. Hold That Megawatt!, The New York Times, 7 January 2011. Discusses AES Energy Storage.
- Wald, Matthew L. Green Blog: Is That Onions You Smell? Or Battery Juice?, The New York Times, 9 May 2012. Discusses vanadium redox battery technology.
- Wald, Matthew L. Green Blog: Cutting the Electric Bill with a Giant Battery, The New York Times, 27 June 2012. Discusses Saft Groupe S.A.
- Wald, Matthew L. Seeking to Start a Silicon Valley for Battery Science, The New York Times, 30 November 2012.
- Wald, Matthew L. From Harvard, a Cheaper Storage Battery, The New York Times, 8 January 2014. Discusses research into flow-batteries utilizing carbon-based molecules called quinones.
- Witkin, Jim. Building Better Batteries for Electric Cars, The New York Times, 31 March 2011, p. F4. Published online 30 March 2011. Discusses rechargeable batteries and lithium ion batteries.
- Witkin, Jim. Green Blog: A Second Life for the Electric Car Battery, The New York Times, 27 April 2011. Describes: ABB; Community Energy Storage for the use of electric vehicle batteries for grid energy storage.
- Woody, Todd. Green Blog: When It Comes to Car Batteries, Moore’s Law Does Not Compute, The New York Times, 6 September 2010. Discusses lithium-air batteries.
- Jang Wook Choi. Promise and reality of post-lithium-ion batteries with high energy densities.
External links
| Wikimedia Commons has media related to Rechargeable batteries. |
- High-performance lithium battery anodes using silicon nanowires
- Scientific American - How Rechargeable Batteries Work
- Electropaedia