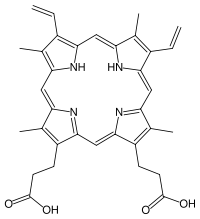
Protoporphyrin IX
 | |
| Identifiers | |
|---|---|
| 553-12-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15430 |
| ChEMBL | ChEMBL267548 |
| ChemSpider | 10469486 |
| ECHA InfoCard | 100.008.213 |
| PubChem | 4971 |
| |
| |
| Properties | |
| C34H34N4O4 | |
| Molar mass | 562.658 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Protoporphyrin IX, in the metabolism of porphyrin, is created by the enzyme protoporphyrinogen oxidase.
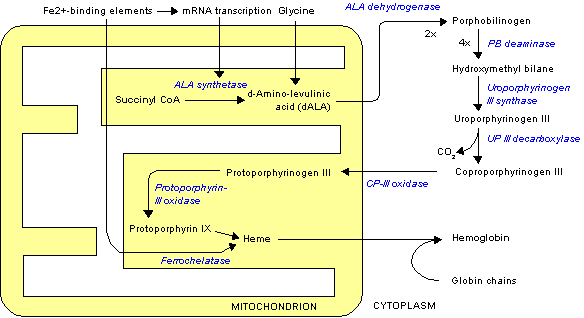
Protoporphyrin IX biosynthesis
Protoporphyrin IX is an important precursor to biologically essential prosthetic groups such as heme, cytochrome c, and chlorophylls. As a result, a number of organisms are able to synthesize this tetrapyrrole from basic precursors such as glycine and succinyl CoA, or glutamate. Despite the wide range of organisms that synthesize protoporphyrin IX the process is largely conserved from bacteria to mammals with a few distinct exceptions in higher plants.[1][2][3]
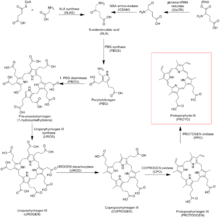
Heme b biosynthesis
In heme biosynthesis, the enzyme ferrochelatase converts it into heme b (i.e. Fe-protoporphyrin IX or protoheme IX).
Chlorophyll biosynthesis
In chlorophyll biosynthesis, the enzyme magnesium chelatase converts it into Mg-protoporphyrin IX.
See also
- Methyl aminolevulinate
- Protoporphyrin for other roles of protoporphyrin IX
- ↑ A. R. Battersby; C. J. R. Fookes; G. W. J. Matcham; E. McDonald (1980). "Biosynthesis of the pigments of life: formation of the macrocycle". Nature. 285: 17–21. doi:10.1038/285017a0.
- ↑ F. J. Leeper (1983). "The biosynthesis of porphyrins, chlorophylls, and vitamin B12". Natural Product Reports. 2: 19–47. doi:10.1039/NP9850200019.
- ↑ G. Layer; J. Reichelt; D. Jahn (2010). "Structure and function of enzymes in heme biosynthesis". Protein Science. 19: 1137–1161. doi:10.1002/pro.405.