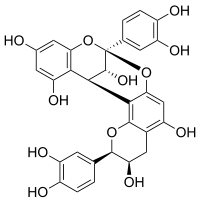
Procyanidin A2
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R,8S,14R,15R)-2,8-bis(3,4-dihydroxyphenyl)-2,3,4,14-tetrahydro-8,14-methanobenzo[7,8][1,3]dioxocino[4,5-h]chromene-3,5,11,13,15-pentaol | |
| Other names
Dimeric catechin Procyanidin A2 Procyanidol A2 Proanthocyanidin A-2 Procyanidin dimer A2 (+)-Proanthocyanidin A2 Epicatechin-(2β→7,4β→8)-epicatechin | |
| Identifiers | |
| 41743-41-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28472 |
| ChemSpider | 110541 |
| PubChem | 124025 |
| |
| |
| Properties | |
| C30H24O12 | |
| Molar mass | 576.51 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Procyanidin A2 is a A type proanthocyanidin.
It is found in avocado,[1] chestnut,[2][3] cranberry juice concentrate,[4] lychee fruit pericarb,[5] peanut[4] skins,[6] Cinchona cortex, cinnamon cortex, Urvillea ulmaceae,[7] and Ecdysanthera utilis.[8]
Synthesis
Procyanidin B2 can be converted into procyanidin A2 by radical oxidation using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals under neutral conditions.[9]
References
- ↑ Proanthocyanidin-A-2 on liberherbarum.com
- ↑ Facino, R. Maffei; Carini, M.; Brambilla, A.; Bombardelli, E.; Morazzoni, P. (1996). "Proanthocyanidin-A2: a new polyphenol". Cosmetics & Toiletries.
- ↑ PMID 21803362
- 1 2 Koerner Jayma, Hsu Victor, Lee Jungmin, Kennedy, James, (2009). "Determination of Proanthocyanidin A2 Content in Phenolic Polymer Isolates by Reversed-Phase High Performance Liquid Chromatography". Journal of Chromatography A. 1216 (9): 1403–1409. doi:10.1016/j.chroma.2008.12.086. PMID 19168185.
- ↑ Sarni-Manchado P, Le Roux E, Le Guerneve C, Lozano Y, Cheynier V. Phenolic composition of litchi fruit pericarp. J Agric Food Chem. 2000;48(12):5995-6002.
- ↑ Hongxiang Lou; Yamazaku Y.; Sasaku T.; Uchida M.; Tanaka H.; Oka S. (1999). "A-type proanthocyanidins from peanut skins". Phytochemistry. 51 (2): 297–308. doi:10.1016/S0031-9422(98)00736-5.
- ↑ Dias, Suziane A.; Cardoso (Gazio), Flávia P.; Santin, Silvana M. O.; Da Costa, Willian F.; Vidotti, Gentil J.; De Souza, Maria Conceição; Sarragiotto, Maria Helena (2009). "Free radical scavenging activity and chemical constituents of Urvillea ulmaceae". Pharmaceutical Biology. 47 (8): 717. doi:10.1080/13880200902933336.
- ↑ Lin, Lie-Chwen; Kuo, Yuh-Chi; Chou, Cheng-Jen (2002). "Immunomodulatory Proanthocyanidins from Ecdysantherautilis". Journal of Natural Products. 65 (4): 505–8. doi:10.1021/np010414l. PMID 11975489.
- ↑ Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Kazunari Kondo, Masaaki Kurihara, Kiyoshi Fukuhara, Takashi Tanaka, Takashi Suzuki, Naoki Miyata and Masatake Toyoda, Tetrahedron Letters, 22 January 2000, Volume 41, Issue 4, Pages 485–488, doi:10.1016/S0040-4039(99)02097-3
Wen LR, Wu D, Jiang YM, Prasad KN, Lin S, Jiang GX, He JR, Zhao MM, Luo W, Yang B. Identification of flavonoids in litchi (Litchi chinensis Sonn.) leaf and evaluation of anticancer activities. Journal of Functional Foods, 2014, 6, 555-563.
This article is issued from Wikipedia - version of the 6/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.