Primordial nuclide
| Nuclear physics |
|---|
 |
| Nucleus · Nucleons (p, n) · Nuclear force · Nuclear structure · Nuclear reaction |
|
Nuclear models and stability |
|
Nucleosynthesis topics Nuclear fusion Processes: Stellar · Big Bang · Supernova Nuclides: Primordial · Cosmogenic · Artificial |
|
Scientists Alvarez · Becquerel · Bethe · A.Bohr · N.Bohr · Chadwick · Cockcroft · Ir.Curie · Fr.Curie · Pi.Curie · Skłodowska-Curie · Davisson · Fermi · Hahn · Jensen · Lawrence · Mayer · Meitner · Oliphant · Oppenheimer · Proca · Purcell · Rabi · Rutherford · Soddy · Strassmann · Szilárd · Teller · Thomson · Walton · Wigner |
In geochemistry and geonuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides are residues from the Big Bang, from cosmogenic sources, and from ancient supernova explosions which occurred before the formation of the Solar System. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present. Only 286 such nuclides are known.
All of the known 254 stable nuclides occur as primordial nuclides, plus another 32 nuclides that have half-lives long enough to have survived from the formation of the Earth. These 32 primordial radionuclides represent isotopes of 28 separate elements.
Cadmium, tellurium, neodymium, samarium and uranium each have two primordial radioisotopes (113
Cd
, 116
Cd
; 128
Te
, 130
Te
; 144
Nd
, 150
Nd
; 147
Sm
, 148
Sm
; and 235
U
, 238
U
).
Due to the age of the Earth of 4.58×109 years (4.6 billion years), this means that the half-life of the given nuclides must be greater than about 1×108 years (100 million years) for practical considerations. For example, for a nuclide with half-life 6×107 years (60 million years), this means 77 half-lives have elapsed, meaning that for each mole (6.02×1023 atoms) of that nuclide being present at the formation of Earth, only 4 atoms remain today.
The shortest-lived primordial nuclides (i.e. nuclides with shortest half-lives) are:
These are the 4 nuclides with half-lives comparable to, or less than, the estimated age of the universe. (In the case of 232Th, it has a half life of more than 14 billion years, slightly longer than the age of the universe.) For a complete list of the 32 known primordial radionuclides, including the next 28 with half-lives much longer than the age of the universe, see the complete list in the section below.
The next longest-living nuclide after the end of the list given in the table is 244
Pu
, with a half-life of 8.08×107 years. It has been reported to exist in nature as a primordial nuclide, although later studies could not detect it.[1] Likewise, the second-longest-lived non-primordial 146
Sm
, was once reported as primordial, but this could not be replicated.[2] Taking into account that all these nuclides must exist since at least 4.6×109 years, meaning survive 57 half-lives, their original number is now reduced by a factor of 257 which equals more than 1017.[3]
Although it is estimated that about 32 primordial nuclides are radioactive (list below), it becomes very difficult to determine the exact total number of radioactive primordials, because the total number of stable nuclides is uncertain. There exist many extremely long-lived nuclides whose half-lives are still unknown. For example, it is known theoretically that all isotopes of tungsten, including those indicated by even the most modern empirical methods to be stable, must be radioactive and can decay by alpha emission, but as of 2013 this could only be measured experimentally for 180
W
.[4] Nevertheless, the number of nuclides with half-lives so long that they cannot be measured with present instruments—and are considered from this viewpoint to be stable nuclides—is limited. Even when a "stable" nuclide is found to be radioactive, the fact merely moves it from the stable to the unstable list of primordial nuclides, and the total number of primordial nuclides remains unchanged.
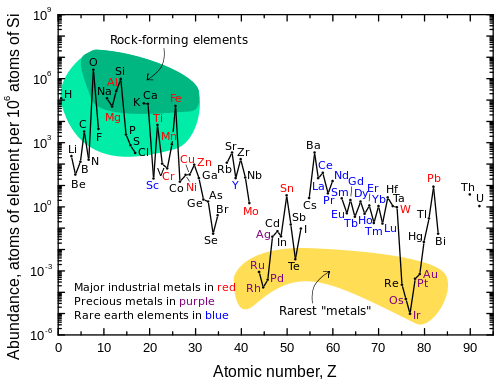
Because primordial chemical elements often consist of more than one primordial isotope, there are only 84 distinct primordial chemical elements. Of these, 80 have at least one observationally stable isotope and four additional primordial elements have only radioactive isotopes.
Naturally occurring nuclides that are not primordial
Some unstable isotopes which occur naturally (such as 14
C
, 3
H
, and 239
Pu
) are not primordial, as they must be constantly regenerated. This occurs by cosmic radiation (in the case of cosmogenic nuclides such as 14
C
and 3
H
), or (rarely) by such processes as geonuclear transmutation (neutron capture of uranium in the case of 239
Pu
). Other examples of common naturally-occurring but non-primordial nuclides are radon, polonium, and radium, which are all radiogenic nuclide daughters of uranium decay and are found in uranium ores. A similar radiogenic series is derived from the long-lived radioactive primordial nuclide thorium-232. All of such nuclides have shorter half-lives than their parent radioactive primordial nuclides.
There are about 51 nuclides which are radioactive and exist naturally on Earth but are not primordial (making a total of fewer than 340 total nuclides to be found naturally on Earth).
Primordial elements
There are 254 stable primordial nuclides and 32 radioactive primordial nuclides, but only 80 primordial stable elements (1 through 82, i.e. hydrogen through lead, exclusive of 43 and 61, technetium and promethium respectively) and three radioactive primordial elements (bismuth, thorium, and uranium). Bismuth's half-life is so long that it is often classed with the 80 primordial stable elements instead, since its radioactivity is not a cause for serious concern. The numbers of elements are smaller, because many primordial elements are represented by more than one primordial nuclide. See chemical element for more information.
Naturally occurring stable nuclides
As noted, these number about 254. For a list, see the article list of stable isotopes. For a complete list noting which of the "stable" 254 nuclides may be in some respect unstable, see list of nuclides and stable isotope. These questions do not impact the question of whether a nuclide is primordial, since all "nearly stable" nuclides, with half-lives longer than the age of the universe, are primordial also.
List of 32 radioactive primordial nuclides and measured half-lives
These 32 primordial nuclides represent radioisotopes of 28 distinct chemical elements (cadmium, neodymium, samarium, tellurium, and uranium each have two primordial radioisotopes). The radionuclides are listed in order of stability, with the longest half-life beginning the list. These radionuclides in many cases are so nearly stable that they compete for abundance with stable isotopes of their respective elements. For three chemical elements, a very long lived radioactive primordial nuclide is found to be the most abundant nuclide for an element that also has a stable nuclide. These unusual elements are tellurium, indium, and rhenium.
The longest has a half-life of 2.2×1024 years, which is 160 million million times the age of the Universe (the latter is about 4.32×1017 s). Only six of these 34 nuclides have half-lives shorter than, or equal to, the age of the universe. Most of the remaining 28 have half-lives much longer. The shortest-lived primordial isotope has a half-life of only 68 million years, less than 1.5% of the age of the Earth and Solar System.
| no | nuclide | energy | half-life (years) | decay mode | decay energy (MeV) | approx ratio half-life to age of universe |
|---|---|---|---|---|---|---|
| 255 | 128Te | 8.743261 | 2.2×1024 | 2 β− | 2.530 | 160 trillion |
| 256 | 136Xe | 8.706805 | 2.165×1021 | 2 β− | 2.462 | 150 billion |
| 257 | 76Ge | 9.034656 | 1.8×1021 | 2 β− | 2.039 | 130 billion |
| 258 | 130Ba | 8.742574 | 1.2×1021 | KK | 2.620 | 90 billion |
| 259 | 82Se | 9.017596 | 1.1×1020 | 2 β− | 2.995 | 8 billion |
| 260 | 116Cd | 8.836146 | 3.102×1019 | 2 β− | 2.809 | 2 billion |
| 261 | 48Ca | 8.992452 | 2.301×1019 | 2 β− | 4.274, .0058 | 2 billion |
| 262 | 96Zr | 8.961359 | 2.0×1019 | 2 β− | 3.4 | 1 billion |
| 263 | 209Bi | 8.158689 | 1.9×1019 | α | 3.137 | 1 billion |
| 264 | 130Te | 8.766578 | 8.806×1018 | 2 β− | .868 | 600 million |
| 265 | 150Nd | 8.562594 | 7.905×1018 | 2 β− | 3.367 | 600 million |
| 266 | 100Mo | 8.933167 | 7.804×1018 | 2 β− | 3.035 | 600 million |
| 267 | 151Eu | 8.565759 | 5.004×1018 | α | 1.9644 | 300 million |
| 268 | 180W | 8.347127 | 1.801×1018 | α | 2.509 | 100 million |
| 269 | 50V | 9.055759 | 1.4×1017 | β+ or β− | 2.205, 1.038 | 10 million |
| 270 | 113Cd | 8.859372 | 7.7×1015 | β− | .321 | 600,000 |
| 271 | 148Sm | 8.607423 | 7.005×1015 | α | 1.986 | 500,000 |
| 272 | 144Nd | 8.652947 | 2.292×1015 | α | 1.905 | 200,000 |
| 273 | 186Os | 8.302508 | 2.002×1015 | α | 2.823 | 100,000 |
| 274 | 174Hf | 8.392287 | 2.002×1015 | α | 2.497 | 100,000 |
| 275 | 115In | 8.849910 | 4.4×1014 | β− | .499 | 30,000 |
| 276 | 152Gd | 8.562868 | 1.1×1014 | α | 2.203 | 8000 |
| 277 | 190Pt | 8.267764 | 6.5×1011 | α | 3.252 | 60 |
| 278 | 147Sm | 8.610593 | 1.061×1011 | α | 2.310 | 8 |
| 279 | 138La | 8.698320 | 1.021×1011 | K or β− | 1.737, 1.044 | 7 |
| 280 | 87Rb | 9.043718 | 4.972×1010 | β− | .283 | 4 |
| 281 | 187Re | 8.291732 | 4.122×1010 | β− or α | .0026, 1.653 | 3 |
| 282 | 176Lu | 8.374665 | 3.764×1010 | β− | 1.193 | 3 |
| 283 | 232Th | 7.918533 | 1.406×1010 | α SF | 4.083 | 1 |
| 284 | 238U | 7.872551 | 4.471×109 | α SF | 4.270 | 0.3 |
| 285 | 40K | 8.909707 | 1.25×109 | β− or K or β+ | 1.311, 1.505, 1.505 | 0.09 |
| 286 | 235U | 7.897198 | 7.04×108 | α SF | 4.679 | 0.05 |
List legends
- no (number)
A running positive integer for reference. These numbers may change slightly in the future since there are 164 nuclides now classified as stable, but which are theoretically predicted to be unstable (see Stable nuclide#Still-unobserved decay), so that future experiments may show that some are in fact unstable. The number starts at 255, to follow the 254 nuclides (or stable isotopes) not yet found to be radioactive.
- nuclide column
Nuclide identifiers are given by their mass number A and the symbol for the corresponding chemical element (implies a unique proton number).
- energy column
The column labeled "energy" denotes the mass of the average nucleon of this nuclide relative to the mass of a neutron (so all nuclides get a positive value) in MeV/c2, formally: mn − mnuclide / A.
- half-life column
All times are given in years
- decay mode column
| α | α decay |
| β− | β− decay |
| K | electron capture |
| KK | double electron capture |
| β+ | β+ decay |
| SF | spontaneous fission |
| 2 β− | double β− decay |
| β+β+ | double β+ decay |
| I | isomeric transition |
| p | proton emission |
| n | neutron emission |
- decay energy column
Multiple values for (maximal) decay energy in MeV are mapped to decay modes in their order.
See also
- Table of nuclides sorted by half-life
- Table of nuclides
- Isotope geochemistry
- Radionuclide
- Mononuclidic element
- Monoisotopic element
- Stable isotope
- List of nuclides
- List of elements by stability of isotopes
- Big Bang nucleosynthesis
References
- ↑ D.C. Hoffman; F.O. Lawrence; J.L. Mewherter; F.M. Rourke (1971). "Detection of Plutonium-244 in Nature". Nature. 234 (5325): 132–134. Bibcode:1971Natur.234..132H. doi:10.1038/234132a0.
- ↑ S. Maji; S. Lahiri; B. Wierczinski; G. Korschinek (2006). "Separation of samarium and neodymium: a prerequisite for getting signals from nuclear synthesis". Analyst. 131 (12): 1332–1334. Bibcode:2006Ana...131.1332M. doi:10.1039/b608157f. PMID 17124541.
- ↑ P.K. Kuroda (1979). "Origin of the elements: pre-Fermi reactor and plutonium-244 in nature". Accounts of Chemical Research. 12 (2): 73–78. doi:10.1021/ar50134a005.
- ↑ "Interactive Chart of Nuclides (Nudat2.5)". National Nuclear Data Center. Retrieved 2009-06-22.