Phthalocyanine Blue BN
| Phthalo Blue | |
|---|---|
|
Phthalocyanine blue pigment powder | |
| Hex triplet | #000f89 |
| sRGBB (r, g, b) | (0, 15, 137) |
| CMYKH (c, m, y, k) | (100, 98, 16, 14) |
| HSV (h, s, v) | (233°, 100%, 54%) |
| Source | The Mother of All HTML Colo(u)r Charts |
|
B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
Phthalocyanine Blue BN, also called Monastral blue, phthalo blue, thalo blue (and others) (CAS 147-14-8, EINECS 205-685-1), is a bright, crystalline, synthetic blue pigment from the group of phthalocyanine dyes.
It was first developed as a pigment in the mid-1930s. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalies and acids. It has the appearance of a blue powder, insoluble in water and most solvents. The anecdotal history of the compound is that a chemist at the ICI phthalimide plant was troubled by blue contamination of the product. This was traced to a by-product formed when the phthalimide reacted with trace amounts of iron from the metal reactor. The chemist took samples of this blue and using sulfuric acid as a solvent, managed to produce a workable pigment. This was converted into the copper centered blue and sold under the trade name Monastral. Difficulty was experienced in forming stable dispersions with the first alpha forms, especially in mixtures with rutile Titanium, where the blue pigment tended to flocculate. The beta form was more stable, as was the improved stabilized alpha form. Today, there are even more isomeric forms available.
Synonyms and trade names
The substance, chemical name (29H,31H-phthalocyaninato(2-)-N29,N30,N31,N32)copper (or copper phthalocyanine),[1] is also known as monastral blue, phthalo blue, helio blue, thalo blue, Winsor blue, phthalocyanine blue, C.I. Pigment Blue 15:2, Copper phthalocyanine blue, Copper tetrabenzoporphyrazine, Cu-Phthaloblue, PB-15, PB-36, C.I. 74160, and British Rail Blue. Numerous other trade names and synonyms exist.[2] The abbreviation "CuPc" is also used.[3]
Applications
Ink
Due to its stability, phthalo blue is also used in inks, coatings, and many plastics. The pigment is insoluble and has no tendency to migrate in the material. It is a standard pigment used in printing ink and the packaging industry.
A common component on the artist's palette, phthalo blue is a cool blue with a bias towards green. It has intense tinting strength and easily overpowers the mix when combined with other colors. It is a transparent staining color and can be applied using glazing techniques.
Industrial production was of the order of 10,000 tonnes pa in the 1980–90s in Japan alone.[2] The pigment is the highest volume pigment produced.[4]
Phthalocyanine Blue BN is also used as a source material for manufacture of Phthalocyanine Green G.
Photovoltaics
Copper phthalocyanine, often referred to as CuPc, is also a leading material used in organic solar cell research. It is well suited for thin film solar cells because of its high chemical stability and uniform growth.[5][6][7] CuPc usually plays the role of the electron donor in donor/acceptor based solar cells. One of the most common donor/acceptor architectures is CuPc/C60 (buckminsterfullerene) which rapidly became a model system for the study of small organic molecules.[8][9] Photon to electron conversion efficiency in such system reaches approximately 5%.
Quantum computing
The compound may also have uses in the development of quantum computing due to the length of time its electrons can exist in a state of quantum superposition.[10] CuPc can be easily processed into a thin film for use in device fabrication, which makes it an attractive qubit candidate.[11]
Structure, reactivity and properties
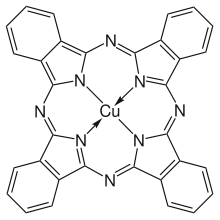
Phthalocyanine blue (C32H16N8Cu) a complex of copper with phthalocyanine. Molecular mass is 576.08,[1] melting point 600 °C (1,112 °F) (with decomposition), the substance is practically insoluble in water (< 0.1 g/100 ml at 20 °C (68 °F)),[1] but soluble in concentrated sulfuric acid.[2] Density of the solid is ~1.6 g/cm3.[2] The color is due to a π-π* electronic transition, with λmax ~ 610 nm.[12]
Crystalline phases
Phthalocyanine compounds belong to the D4h point group[13][14] and may crystallize in various forms called polymorphs. To date, four different polymorphs were identified:[15][16] phases α, β, ɣ and x. The two most common structures in CuPc are the β phase and the metastable α phase. Those phases can be distinguished by the overlap of their neighboring molecules. The α phase has a larger overlap and thus, a smaller Cu-Cu spacing (~ 3.8 Å) compare to the β phase (~ 4.8 Å).[17]
Toxicity and hazards
The compound is non-biodegradeable, but not toxic to fish or plants.[2] No specific dangers have been associated with this compound.[18] Oral LD50 in mammals is estimated to be greater than 5 g per kg, with no ill effects found at that level of ingestion,[2] for chronic ingestion levels of concern were found to occur at 0.2 g/kg or greater.[2] There is no evidence for carcinogenic effects yet known.[2] There is some evidence that exposure to phthalocyanines can cause serious birth defects in developing embryos.[19]
See also
- Phthalocyanine Green G
- British Rail corporate liveries § Rail Blue - use of the pigment as the standard livery for British Rail trains from 1965 onwards.
- The Joy of Painting - oil paint based on the pigment was frequently used on the show.
- List of colors
References
- 1 2 3 Copper phthalocyanine chemblink.com
- 1 2 3 4 5 6 7 8 COPPER PHTHALOCYANINE, CAS No.: 147-14-8 inchem.org
- ↑ e.g. Structural and Transport Properties of Copper Phthalocyanine (CuPc) Thin Films www.egmrs.org
- ↑ Industrial applications of phthalocyanines. Author : Peter Gregory, Journal of Porphyrins and Phthalocyanines (JPP) Vol 4 Issue 4 Year 2000 via worldscinet.com
- ↑ Szybowicz, M (October 2004). "High temperature study of FT-IR and Raman scattering spectra of vacuum deposited CuPc thin films". Journal of Molecular Structure. 704: 107–113. doi:10.1016/j.molstruc.2004.01.053.
- ↑ Wojdyla, Michal; Derkowska, Beata; Bala, Waclaw Bala (2005). "Lock-in phase analysis of copper phthalocyanine photoabsorption spectrum". Optica Applicata. 35 (3): 561–571.
- ↑ Bala, M; Wojdyla, M; Rebarz, M; Szybowic, M; Drozdowski, M; Grodzicki, A; Piszczek, P (2009). "Influence of central metal atom in MPc ( M = Cu , Zn , Mg , Co ) on Raman, FT-IR, absorbance, reflectance, and photoluminescence spectra". J. Optoelectron. Adv. M. 11 (3): 264–269.
- ↑ Askat E, Jailaubekov (2013). "Hot charge-transfer excitons set the time limit for charge separation at donor/acceptor interfaces in organic photovoltaics". Nature Materials. 10: 66–73. doi:10.1038/nmat3500.
- ↑ Xin, Li (January 2013). "CuPc/C60 bulk heterojunction photovoltaic cells with evidence of phase segregation". Organic Electronics. 14: 250–254. doi:10.1016/j.orgel.2012.10.041.
- ↑ Warner, Marc; et al. (October 26, 2013). "New Material for Quantum Computing Discovered Out of the Blue". Nature. Retrieved November 3, 2013.
- ↑ Quenqua, Douglas (November 4, 2013). "A Key to Quantum Computing, Close to Home". The New York Times.
- ↑ H. S. Rzepa, www.ch.imperial.ac.uk/rzepa/blog/?p=3641, Accessed: 2011-03-08. (Archived by WebCite® at http://www.webcitation.org/5x2Q0jeBj)
- ↑ James H., Sharp; Martin, Abkowitz (1973). "Dimeric Structure of a Copper Phthalocyanine Polymorph". J. Phys. Chem. 77 (11): 477–481. doi:10.1021/j100623a012.
- ↑ P. N., Day; Zhiqiang, Wang; R, Pachter (1998). "Calculation of the structure and absorption spectra of phthalocyanines in the gas-phase and in solution". Journal of Molecular Structure : (Theochem). 455 (1): 33–50. doi:10.1016/S0166-1280(98)00238-3.
- ↑ Jacques M., Assour (1965). "On the Polymorphic Modifications of Phthalocyanines". J. Phys. Chem. 69 (7): 2295–2299. doi:10.1021/j100891a026.
- ↑ A.K., Hassan; R.D., Gould (2006). "Structural Studies of Thermally Evaporated Thin Films of Copper Phthalocyanine". Physica Status Solidi (a). 132 (1): 91–101. doi:10.1002/pssa.2211320110.
- ↑ Amy C, Cruickshank; Christian J, Dotzler; Salahud, Din; Sandrine, Heutz; Michael F, Toney; Mary P, Ryan (2012). "The crystalline structure of copper phthalocyanine films on ZnO(1100)". Journal of the American Chemical Society. 134 (35): 14302–14305. doi:10.1021/ja305760b.
- ↑ Safety data sheet cornelius.co.uk
- ↑ "Sulphonated phthalocyanine induced caudal malformative syndrome in the chick embryo." (Abstract), Sandor S, Prelipceanu O, and Checiu I., U.S. National Library of Medicine National Institutes of Health
External links
- Discovery of a new pigment - "Monastral blue" colorantshistory.org
- Patrick Linstead talking about phthalocyanine Imperial College London, Chemistry department