Mauveine

Mauveine, also known as aniline purple and Perkin's mauve, was the first synthetic organic chemical dye,[1][2] discovered serendipitously in 1856. It is also among the first chemical dyes to have been mass-produced.
Chemistry
Mauveine is a mixture of four related aromatic compounds differing in number and placement of methyl groups. Its organic synthesis involves dissolving aniline, p-toluidine, and o-toluidine in sulfuric acid and water in a roughly 1:1:2 ratio, then adding potassium dichromate.[3]
Mauveine A (C26H23N4+X−) incorporates 2 molecules of aniline, one of p-toluidine, and one of o-toluidine. Mauveine B (C27H25N4+X−) incorporates one molecule each of aniline, p-toluidine, and o-toluidine. In 1879, Perkin showed mauveine B related to safranines by oxidative/reductive loss of the p-tolyl group.[4] In fact, safranine is a 2,8-dimethyl phenazinium salt, whereas the parasafranine produced by Perkin is presumed[5] to be the 1,8-(or 2,9) dimethyl isomer.
The molecular structure of mauveine proved difficult to determine, finally being identified in 1994.[6] In 2007, two more were isolated and identified: mauveine B2, an isomer of mauveine B with methyl on different aryl group, and mauveine C, which has one more p-methyl group than mauveine A.[7]
-

skeletal formula of mauveine A
-

skeletal formula of mauveine B
-

skeletal formula of mauveine B2
-
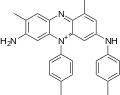
skeletal formula of mauveine C
In 2008, additional mauveines and pseudomauveines were discovered, bringing the total number of these compounds up to 12.[8]
History
.jpg)
In 1856, William Henry Perkin, then age 18, was given a challenge by his professor, August Wilhelm von Hofmann, to synthesize quinine. In one attempt, Perkin oxidized aniline using potassium dichromate, whose toluidine impurities reacted with the aniline and yielded a black solid—suggesting a "failed" organic synthesis. Cleaning the flask with alcohol, Perkin noticed purple portions of the solution.
Suitable as a dye of silk and other textiles, it was patented by Perkin, who the next year opened a dyeworks mass-producing it at Greenford on the banks[9] of the Grand Union Canal in London. It was originally called aniline purple or Tyrian purple, the name of an ancient natural dye derived from mollusks.[10] In 1859, it attained the name mauve in England via the French name for the mallow flower, and chemists later called it mauveine.[10] By 1870, its great demand succumbed to newer synthetic colors in the synthetic dye industry launched by mauveine.
In the early 20th century, the U.S. National Association of Confectioners permitted mauveine as a food coloring with a variety of equivalent names: rosolan, violet paste, chrome violet, anilin violet, anilin purple, Perkin's violet, indisin, phenamin, purpurin, tyralin, Tyrian purple, and lydin.[11]
Laborers in the aniline dye industry were later found at increased risk of bladder cancer, specifically transitional cell carcinoma,[12] yet by the 1950s, the synthetic dye industry helped transform medicine,[13] including cancer treatment.[14] (See "Aniline", section "History".)
References
- ↑ Hubner K (2006). "History – 150 Years of mauveine". Chemie in unserer Zeit. 40 (4): 274–275. doi:10.1002/ciuz.200690054.
- ↑ Anthony S. Travis (1990). "Perkin's Mauve: Ancestor of the Organic Chemical Industry". Technology and Culture. 31 (1): 51–82. doi:10.2307/3105760. JSTOR 3105760.
- ↑ A Microscale Synthesis of Mauve Scaccia, Rhonda L.; Coughlin, David; Ball, David W. J. Chem. Educ. 1998 75 769 Abstract
- ↑ Perkin, W. H. "On mauveine and allied colouring matters". J. Chem. Soc. Trans. 1879: 717–732. doi:10.1039/CT8793500717.
- ↑ Website source: ch.ic.ac.uk Link
- ↑ Meth-Cohn, O.; Smith, M. "What did W. H. Perkin actually make when he oxidised aniline to obtain mauveine?". J. Chem. Soc. Perkin 1. 1994: 5–7. doi:10.1039/P19940000005.
- ↑ J. Seixas de Melo, S. Takato, M. Sousa, M. J. Melo and A. J. Parola Revisiting Perkin's dye(s): The spectroscopy and photophysics of two new mauveine compounds (B2 and C) Chem. Commun. 2007; 2624–26 doi:10.1039/b618926a
- ↑ A Study in Mauve: Unveiling Perkin!s Dye in Historic Samples, M. M. Sousa, M. J. Melo, A. J. Parola, P. J. T. Morris, H. S. Rzepa, and J. S. Seixas de Melo Chem. Eur. J., 2008, 14, 8507– 8513, doi:10.1002/chem.200800718
- ↑ Google Earth location: Download
- 1 2 Matthew,, H.C.G.; Brian Howard Harrison (2004). Oxford Dictionary of National Biography: In Association with the British Academy. Oxford University Press. ISBN 0-19-861393-8.
- ↑ Leffmann, Henry; William Beam (1901). Select Methods in Food Analysis. Philadelphia: P. Blakiston's Son & Co.
- ↑ Cartwright, R.A. (1983). "Historical and modern epidemiological studies on populations exposed to N-substituted aryl compounds" (PDF). Environmental Health Perspectives. 49: 13–19. doi:10.1289/ehp.834913. PMC 1569142
 . PMID 6339220.
. PMID 6339220. - ↑ John E Lesch, The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine (New York: Oxford University Press, 2007), pp 202–3.
- ↑ D J Th Wagener, The History of Oncology (Houten: Springer, 2009), pp 150–1.
External links
- Perkin anniversary website
- Rotatable 3D models of mauveine are available using Jmol