Peptoid
Peptoids, or poly-N-substituted glycines, are a class of peptidomimetics whose side chains are appended to the nitrogen atom of the peptide backbone, rather than to the α-carbons (as they are in amino acids).
Chemical structure and synthesis
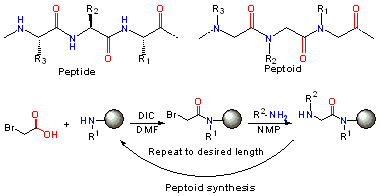
In peptoids the side chain is connected to the nitrogen of the peptide backbone, instead of the α-carbon as in peptides. Notably, peptoids lack the amide hydrogen which is responsible for many of the Secondary structure elements in peptides and proteins. Peptoids were first invented by Reyna J. Simon, Paul Bartlett and Daniel V. Santi to mimic protein/peptide products to aid in the discovery of protease-stable small molecule drugs.[1][2]
Following the sub-monomer protocol originally created by Ron Zuckermann,[3] each residue is installed in two steps: acylation and displacement. In the acylation step a haloacetic acid, typically bromoacetic acid activated by diisopropylcarbodiimide reacts with the amine of the previous residue. In the displacement step (a classical SN2 reaction), an amine displaces the halide to form the N-substituted glycine residue. The submonomer approach allows the use of any commercially available or synthetically accessible amine with great potential for Combinatorial chemistry.
Unique characteristics
Like D-Peptides and β peptides, peptoids are completely resistant to proteolysis,[4] and are therefore advantageous for therapeutic applications where proteolysis is a major issue. Since secondary structure in peptoids does not involve hydrogen bonding, it is not typically denatured by solvent, temperature, or chemical denaturants such as urea (see details below).
Notably, since the amino portion of the amino acid results from the use of any amine, thousands of commercially available amines can be used to generate unprecedented chemical diversity at each position at costs far lower than would be required for similar peptides or peptidomimetics. To date, at least 230 different amines have been used as side chains in peptoids.[5]
Structure
Peptoid oligomers are known to be conformationally unstable, due to the flexibility of the main-chain methylene groups and the absence of stabilizing hydrogen bond interactions along the backbone. Nevertheless, through the choice of appropriate side chains it is possible to form specific steric or electronic interactions that favour the formation of stable secondary structures like helices,[6] especially peptoids with C-α-branched side chains are known to adopt structure analogous to polyproline I helix.[7] Different strategies have been employed to predict and characterize peptoid secondary structure, with the ultimate goal of developing fully folded peptoid protein structures.[8] The cis/trans amide bond isomerization still leads to a conformational heterogeneity which doesn’t allow for the formation of homogeneous peptoid foldamers.[9] Nonetheless scientists were able to find trans-inducer N-Aryl side chains promoting polyproline type II helix,[10] and strong cis-inducer such as bulky naphtylethyl[11] and tert-butyl[12] side chains. It was also found that n→π* interactions can modulate the ratio of cis/trans amide bond conformers,[13] until reaching a complete control of the cis conformer in the peptoid backbone using a functionalizable triazolium side chain.[14]
Applications
The first demonstration of the use of peptoids was in screening a combinatorial library of diverse peptoids which yielded novel high-affinity ligands for 7-transmembrane G-protein-couple receptors.[15]
Peptoids have been developed as candidates for a range of different biomedical applications,[16][17] including antimicrobial agents and synthetic lung surfactants,[18] as well as ligands for various proteins including Src Homology 3 (SH3 domain),[19] Vascular Endothelial Growth Factor (VEGF) receptor 2,[20] and antibody Immunoglobulin G biomarkers for the identification of Alzheimer's disease.[21]
Due to their advantageous characteristics as described above, peptoids are also being actively developed for use in nanotechnology,[22] an area in which they may play an important role.[23]
See also
References
- ↑ Reyna J Simon, Robert S Kania, Ronald N Zuckermann, Verena D Huebner, David A Jewell, Steven Banville, Simon Ng, Liang Wang, Steven Rosenberg, Charles K Marlowe, David C Spellmeyer, Ryoying Tan, Alan D Frankel, Daniel V Santi, Fred E Cohen, and Paul A Bartlett, "Peptoids: a modular approach to drug discovery" Proceedings of the National Academy of Sciences USA, (1992), 89(20), 9367-9371
- ↑ Reyna J Simon, Paul A Bartlett, Daniel V Santi, "Peptoid Mixtures", US Patent 5,811,387, Sept 22, 1998
- ↑ Ronald N. Zuckermann, Janice M. Kerr, Stephen B. H. Kent, Walter H. Moos, Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis Journal of the American Chemical Society, (1992), 114(26), 10646-10647 doi:10.1021/ja00052a076
- ↑ Susan M. Miller, Reyna J. Simon, Simon Ng, Ronald N. Zuckermann, Janice M. Kerr, Walter H. Moos, Comparison of the Proteolytic Susceptibilities of Homologous L-Amino Acid, D-Amino Acid, and N-Substituted Glycine Peptide and Peptoid Oligomers Drug. Dev. Res. (1995), 35, 20-32
- ↑ Adrian S. Culf and Rodney J. Ouellette, Solid-Phase Synthesis of N-Substituted Glycine Oligomers (α-Peptoids) and Derivatives Molecules (2010), 15, 5282-5335 doi:10.3390/molecules15085282
- ↑ Kirshenbaum K, Barron AE, Goldsmith RA, Armand P, Bradley EK, Truong KTV, Dill KA, Cohen FE, Zuckermann RN: Sequence specific polypeptoids: a diverse family of heteropolymers with stable secondary structure., Proc Natl Acad Sci U S A, 1998, 95:4303-4308
- ↑ Philippe Armand, Kent Kirshenbaum, Richard A. Goldsmith, Shauna Farr-Jones, Annelise E. Barron, Kiet T. V. Truong, Ken A. Dill, Dale F. Mierke, Fred E. Cohen, Ronald N. Zuckermann, and Erin K. Bradley, "NMR determination of the major solution conformation of a peptoid pentamer with chiral side chains", Proceedings of the National Academy of Sciences (95(8)): 4309–4314
- ↑ Modi Wetzler and Annelise E. Barron Progress in the de novo design of structured peptoid protein mimic, Biopolym. Pept. Sci. (2011) doi:10.1002/bip.21621
- ↑ Barney Yoo and Kent Kirshenbaum, Peptoid architectures: elaboration, actuation, and application, Current Opinion in Chemical Biology, 2008, 12:714–721
- ↑ Shah, N. H.; Butterfoss, G. L.; Nguyen, K.; Yoo, B.; Bonneau, R.; Rabenstein, D. L.; Kirshenbaum, K., Oligo(N-aryl glycines): A New Twist on Structured Peptoids, J. Am. Chem. Soc. 2008, 130, 16622-16632 article doi:10.1021/ja804580n
- ↑ Stringer, J. R.; Crapster, J. A.; Guzei, I. A.; Blackwell, H. E., Extraordinarily Robust Polyproline Type I Peptoid Helices Generated via the Incorporation of α-Chiral Aromatic N-1-Naphthylethyl Side Chains J. Am. Chem. Soc. 2011, 133, 15559-15567 article doi:10.1021/ja204755p
- ↑ O. Roy, C. Caumes, Y. Esvan, C. Didierjean, S. Faure, C. Taillefumier, The tert-Butyl Side Chain: A Powerful Means to Lock Peptoid Amide Bonds in the Cis Conformation, Org. Lett., 2013, 2246-2249 article doi:10.1021/ol400820y
- ↑ Benjamin C. Gorske, Joseph R. Stringer, Brent L. Bastian, Sarah A. Fowler, Helen E. Blackwell, New Strategies for the Design of Folded Peptoids Revealed by a Survey of Noncovalent Interactions in Model Systems, J. Am. Chem. Soc., 2009, 16555–16567 article doi:10.1021/ja907184g
- ↑ Cécile Caumes, Olivier Roy, Sophie Faure, and Claude Taillefumier, The Click Triazolium Peptoid Side Chain: A Strong cis-Amide Inducer Enabling Chemical Diversity, J. Am. Chem. Soc., 2012, 9553−9556 article doi:10.1021/ja302342h
- ↑ Ronald N Zuckermann, Eric J Martin, David C Spellmeyer, Gregory B Stauber, Kevin R Shoemaker, Janice M Kerr, Gianine M Figliozzi, Dane A Goff, Michael A Siani, Reyna J Simon, et al. "Discovery of nanomolar ligands for 7-transmembrane G-protein-coupled receptors from a diverse N-(substituted)glycine peptoid library," J Med Chem (1994) 37(17):2678-85.
- ↑ Sarah A. Fowler, Helen E. Blackwell, Structure-function relationships in peptoids: recent advances toward deciphering the structural requirements for biological function, Org. Biomol. Chem. (2009), 7(8), 1508-1524 doi:10.1039/B817980H
- ↑ Ronald N. Zuckermann, Thomas Kodadek Peptoids as Potential Therapeutics, Curr. Opin. Mol. Ther. (2009), 11(3), 299-307
- ↑ Nathan J. Brown, Jan Johansson, Annelise E. Barron, Biomimicry of Surfactant Protein C Accounts of Chemical Research, 41(10), 1409-1417 doi:10.1021/ar800058t
- ↑ Jack T. Nguyen, Christoph W. Turck, Fred E. Cohen, Ronald N. Zuckermann, Wendell A. Lim, Exploiting the basis of proline recognition by SH3 and WW domains: design of N-substituted inhibitors, Science (1998), 282(5396), 2088-2092 doi:10.1126/science.282.5396.2088
- ↑ D. Gomika Udugamasooriya, Sean P. Dineen, Rolf A. Brekken and Thomas Kodadek, A Peptoid "Antibody Surrogate" That Antagonizes VEGF Receptor 2 Activity and the proteosome regulatory particle, Journal of the American Chemical Society, (2008), 130(17), 5744-5752, doi:10.1021/ja711193x
- ↑ M. Muralidhar Reddy, Rosemary Wilson, Johnie Wilson, Steven Connell, Anne Gocke, Linda Hynan, Dwight German, Thomas Kodadek, Identification of candidate IgG biomarkers for Alzheimer's disease via combinatorial library screening, Cell (2011), 144(1), 132-142 doi:10.1016/j.cell.2010.11.054 PMID 21215375
- ↑ Ki Tae Nam, Sarah A. Shelby, Philip H. Choi, Amanda B. Marciel, Ritchie Chen, Li Tan, Tammy K. Chu, Ryan A. Mesch, Byoung-Chul Lee, Michael D. Connolly, Christian Kisielowski, Ronald N. Zuckermann Free-floating ultrathin two-dimensional crystals from sequence-specific peptoid polymers, Nat. Mater. (2010), 9(5), 464-460 doi:10.1038/nmat2742
- ↑ K. Eric Drexler, Peptoids at the 7th summit: Toward macromolecular systems engineering Biopolym. Pept. Sci. (2011)doi:10.1002/bip.21623