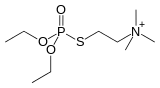
Organothiophosphate
Organothiophosphates are a subclass of organophosphorus compounds. Many of these compounds are used as pesticides, some have medical applications, and some are used as oil additives.[1] They generally have the chemical formula (RO)3PS, [(RO)2P(S)O]−, R(RO)2PS, etc.
| Selected organothiophosphates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Oligonucleotide phosphorothioates (OPS) are modified oligonucleotides where one of the oxygen atoms in the phosphate moiety is replaced by sulfur. These compounds are the basis of antisense therapy, e.g., the drugs fomivirsen (Vitravene), Oblimersen, Alicaforsen, and Mipomersen (Kynamro).[2]
Further examples of these include:
Variants with P=O double bonds were developed as insecticides because of their reduced mammalian toxicity. The phosphorothioate P=S bond is converted to the toxic P=O bond in the target insect. Similar oxidative conversion in mammals is slower, conferring lower toxicity in mammals.
Structure and chemical synthesis
Generally these compounds feature tetrahedral phosphorus(V) centers. Classically, thiophosphates would include a P=S double bond as illustrated by Malathion. The terminology is used loosely and thiophosphates include P-S single bonds as illustrated by the drug Amifostine.
They are conceptually derived from the inorganic thiophosphates (PO4-xS3−
x). In fact, many are prepared via the intermediacy diorganodithiophosphoric acids, which are prepared by treating phosphorus pentasulfide with alcohols:[1]
- P2S5 + 4 ROH → 2 (RO)2PS2H + H2S
Dimethyl dithiophosphoric acid and diethyl dithiophosphoric acid are obtained in this way. The former is a precursor to Malathion.
External links
- Organothiophosphorus Compounds at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- 1 2 J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a19_545.pub2
- ↑ Kurreck, J., "Antisense technologies. Improvement through novel chemical modifications", European Journal of Biochemistry 2003, 270, 1628-1644.doi:10.1046/j.1432-1033.2003.03555.x