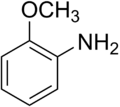
o-Anisidine
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methoxyaniline | |||
| Other names
o-Anisidine (no longer recommended[1]) 2-Anisidine ortho-Aminoanisole o-Methoxyaniline 2-Methoxy-1-aminobenzene 2-Methoxyphenylamine | |||
| Identifiers | |||
| 90-04-0 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEMBL | ChEMBL1612004 | ||
| ChemSpider | 13860775 | ||
| ECHA InfoCard | 100.001.785 | ||
| EC Number | 201-963-1 | ||
| KEGG | C19191 | ||
| UN number | 2431 | ||
| |||
| |||
| Properties[2] | |||
| C7H9NO | |||
| Molar mass | 123.16 g·mol−1 | ||
| Appearance | Yellow liquid, turns brown upon exposure to air | ||
| Density | 1.0923 g/cm3 | ||
| Melting point | 6.2 °C (43.2 °F; 279.3 K) | ||
| Boiling point | 224 °C (435 °F; 497 K) | ||
| 1.5 g/100 ml | |||
| Solubility | soluble in ethanol, diethyl ether, acetone, benzene | ||
| Hazards | |||
| Main hazards | potential occupational carcinogen[3] | ||
| EU classification (DSD) |
Toxic (T) Carc. Cat. 2 Muta. Cat. 3 | ||
| R-phrases | R45, R23/24/25, R68 | ||
| S-phrases | S53, S45 | ||
| NFPA 704 | |||
| Flash point | 118 °C (244 °F; 391 K) (open cup) | ||
| 415 °C (779 °F; 688 K) | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
2000 mg/kg (rat, oral) 1400 mg/kg (mouse, oral) 870 mg/kg (rabbit, oral)[4] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 0.5 mg/m3 [skin][3] | ||
| REL (Recommended) |
: Ca TWA 0.5 mg/m3 [skin][3] | ||
| IDLH (Immediate danger) |
50 mg/m3[3] | ||
| Related compounds | |||
| Related compounds |
m-Anisidine p-Anisidine | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
o-Anisidine (2-anisidine) is a yellow liquid with a melting point of −1 to 5 °C and a density of 1.09 g/cm3. The vapor pressure is 0.05 mbar at 20 °C but increases greatly with temperature. It has an aromatic smell and is well absorbed by inhalation, oral ingestion and skin contact. o-Anisidine is a very toxic agent that causes blood, enzyme and nerve damage with cyanosis and the danger of suffocation.[3] The agent is an experimental carcinogen and is strongly suspected to be a human carcinogen also. o-Anisidine has dangerous pollutant properties for water. Non-wastewaters from the production of dyes containing o-Anisidine are listed as RCRA hazardous waste, with the code K181.[5] o-Anisidine is used in the manufacture of dyes. Workers in the dye industry may be occupationally exposed to it. Acute (short-term) exposure to o-anisidine results in skin irritation in humans. Workers exposed to o-anisidine by inhalation for 6 months developed headaches, vertigo, and effects on the blood. Animal studies have reported effects on the blood from chronic (long-term) dermal exposure to o-anisidine. No information is available on the reproductive, developmental, or carcinogenic effects of o-anisidine in humans. Animal studies have reported tumors of the urinary bladder from oral exposure to o-anisidine. EPA has not classified o-anisidine for carcinogenicity. The International Agency for Research on Cancer (IARC) has classified o-anisidine as a Group 2B, possible human carcinogen.[6]
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 669. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The names ‘toluidine’, ‘anisidine’, and ‘phenetidine’ for which o-, m-, and p- have been used to distinguish isomers, and ‘xylidine’ for which numerical locants, such as 2,3-, have been used, are no longer recommended, nor are the corresponding prefixes ‘toluidine’, ‘anisidino’, ‘phenetidine’, and ‘xylidino’.
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-98. ISBN 0-8493-0462-8..
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0034". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "o-Anisidine". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ http://www.epa.gov/osw/hazard/wastetypes/wasteid/dyes/index.htm
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/o-anisidine#section=Top
External links
- International Chemical Safety Card 0970
- "NIOSH Pocket Guide to Chemical Hazards #0034". National Institute for Occupational Safety and Health (NIOSH).