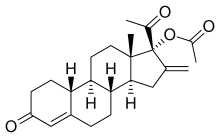
Segesterone acetate
 | |
| Clinical data | |
|---|---|
| Routes of administration | Subcutaneous implant, vaginal ring, transdermal patch[1] |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10% (oral)[1][2] |
| Protein binding | Albumin[1][3] |
| Biological half-life | 1–2 hours (oral)[1] |
| Identifiers | |
| |
| Synonyms | 16-Methylene-17α-acetoxy-19-norprogesterone; 16-Methylene-17α-acetoxy-19-norpregn-4-ene-3,20-dione |
| CAS Number | 7759-35-5 |
| PubChem (CID) | 108059 |
| ChemSpider | 97161 |
| Chemical and physical data | |
| Formula | C23H30O4 |
| Molar mass | 370.482 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Segesterone acetate (USAN)[4][5] (brand name Nestorone; former developmental code names ST-1435, AC-6844, CS-0411),[6] known more commonly simply as nestorone (or nestoron), and also known as elcometrine,[7] is a steroidal progestin of the 19-norprogesterone group which is used as a hormonal contraceptive in several South American countries.[1] Segesterone acetate is the acetate ester of segesterone, which was never marketed, but is an active metabolite of segesterone acetate.[8] Segesterone acetate is only weakly active orally, and is instead given as a subcutaneous implant.[9] It is more than 100-fold times as potent when delivered subcutaneously relative to orally.[1]
Segesterone acetate acts primarily as a high-affinity agonist of the progesterone receptor.[3] It does not bind significantly tot he androgen receptor, estrogen receptor, or mineralocorticoid receptor.[3][10] Segesterone acetate does however have some affinity for the glucocorticoid receptor, where it appears to act as an agonist, but it does not appear to produce any glucocorticoid side effects unless used at high doses.[3][1][11] Segesterone acetate does not bind to sex hormone-binding globulin, and is instead bound to serum albumin.[1][3]
Segesterone acetate is not available in the United States.[1]
See also
References
- 1 2 3 4 5 6 7 8 9 Thomas L. Lemke; David A. Williams; Victoria F. Roche; S. William Zito (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 1403. ISBN 978-1-60913-345-0. Retrieved 13 September 2012.
- ↑ A.R. Genazzani (15 May 2001). Hormone Replacement Therapy and Cardiovascular Disease: The Current Status of Research and Practice. CRC Press. pp. 95–. ISBN 978-1-84214-038-3.
- 1 2 3 4 5 Kuhl, H (2009). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (sup1): 3–63. doi:10.1080/13697130500148875. ISSN 1369-7137.
- ↑ http://www.ama-assn.org/resources/doc/usan/x-pub/segesterone-acetate.pdf
- ↑
- ↑ http://scientonline.org/open-access/nestorone-a-new-hope-for-gynecologists-andrologists-and-neurologists.pdf
- ↑ Martin Negwer; Hans-Georg Scharnow (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. ISBN 978-3-527-30247-5. Retrieved 13 September 2012.
- ↑ Prasad PV, Bashir M, Sitruk-Ware R, Kumar N (2010). "Single-dose pharmacokinetics of Nestorone, a potential female-contraceptive". Steroids. 75 (3): 252–64. doi:10.1016/j.steroids.2009.12.011. PMID 20064539.
- ↑ Sanjay Rajagopalan; Debabrata Mukherjee; Emile R. Mohler (31 August 2004). Manual of Vascular Diseases. Lippincott Williams & Wilkins. p. 803. ISBN 978-0-7817-4499-7. Retrieved 13 September 2012.
- ↑ Hussain R, El-Etr M, Gaci O, et al. (October 2011). "Progesterone and Nestorone facilitate axon remyelination: a role for progesterone receptors". Endocrinology. 152 (10): 3820–31. doi:10.1210/en.2011-1219. PMID 21828184.
- ↑ Kumar N, Koide SS, Tsong Y, Sundaram K (2000). "Nestorone: a progestin with a unique pharmacological profile". Steroids. 65 (10-11): 629–36. doi:10.1016/S0039-128X(00)00119-7. PMID 11108869.