Two-photon excitation microscopy
Two-photon excitation microscopy is a fluorescence imaging technique that allows imaging of living tissue up to about one millimetre in depth. It differs from traditional fluorescence microscopy, in which the excitation wavelength is shorter than the emission wavelength, as the wavelengths of the two exciting photons are longer than the wavelength of the resulting emitted light. Two-photon excitation microscopy typically uses near-infrared excitation light which can also excite fluorescent dyes. However, for each excitation, two photons of infrared light are absorbed. Using infrared light minimizes scattering in the tissue. Due to the multiphoton absorption, the background signal is strongly suppressed. Both effects lead to an increased penetration depth for these microscopes. Two-photon excitation can be a superior alternative to confocal microscopy due to its deeper tissue penetration, efficient light detection, and reduced phototoxicity.[1]
Concept
Two-photon excitation employs two-photon absorption, a concept first described by Maria Goeppert-Mayer (1906–1972) in her doctoral dissertation in 1931,[2] and first observed in 1961 in a CaF2:Eu2+ crystal using laser excitation by Wolfgang Kaiser.[3] Isaac Abella showed in 1962 in cesium vapor that two-photon excitation of single atoms is possible.[4]
Two photon excited fluorescence microscopy has similarities to confocal laser scanning microscopy. Both use focused laser beams scanned in a raster pattern to generate images, and both have an optical sectioning effect. Unlike confocal microscopes, multiphoton microscopes do not contain pinhole apertures that give confocal microscopes their optical sectioning quality. The optical sectioning produced by multiphoton microscopes is a result of the point spread function: the multiphoton point spread function is typically dumbbell-shaped (longer in the x-y plane), compared to the upright rugby-ball shaped point spread function of confocal microscopes. The concept of two-photon excitation is based on the idea that two photons of comparably lower energy than needed for one photon excitation can also excite a fluorophore in one quantum event. Each photon carries approximately half the energy necessary to excite the molecule. An excitation results in the subsequent emission of a fluorescence photon, typically at a higher energy than either of the two excitatory photons. The probability of the near-simultaneous absorption of two photons is extremely low. Therefore, a high flux of excitation photons is typically required, usually from a femtosecond laser. The purpose of employing the two-photon effect is that the axial spread of the point spread function is substantially lower than for single-photon excitation. As a result, the resolution along the z dimension is improved, allowing for thin optical sections to be cut. In addition, in many interesting cases the shape of the spot and its size can be designed to realize specific desired goals.[5] The longer wavelength, lower energy (typically infrared) excitation lasers of multiphoton microscopes are well-suited to use in imaging live cells as they cause less damage than short-wavelength lasers typically used for single-photon excitation, so cells may be observed for longer periods with fewer toxic effects.
The most commonly used fluorophores have excitation spectra in the 400–500 nm range, whereas the laser used to excite the two-photon fluorescence lies in the ~700–1000 nm (infrared) range. If the fluorophore absorbs two infrared photons simultaneously, it will absorb enough energy to be raised into the excited state. The fluorophore will then emit a single photon with a wavelength that depends on the type of fluorophore used (typically in the visible spectrum). Because two photons are absorbed during the excitation of the fluorophore, the probability for fluorescent emission from the fluorophores increases quadratically with the excitation intensity. Therefore, much more two-photon fluorescence is generated where the laser beam is tightly focused than where it is more diffuse. Effectively, excitation is restricted to the tiny focal volume (~1 femtoliter), resulting in a high degree of rejection of out-of-focus objects. This localization of excitation is the key advantage compared to single-photon excitation microscopes, which need to employ additional elements such as pinholes to reject out-of-focus fluorescence. The fluorescence from the sample is then collected by a high-sensitivity detector, such as a photomultiplier tube. This observed light intensity becomes one pixel in the eventual image; the focal point is scanned throughout a desired region of the sample to form all the pixels of the image. The scan head is typically composed of two mirrors, the angles of which can be rapidly altered with a galvanometer.
Development
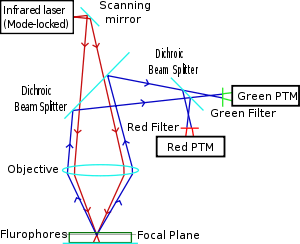
Two-photon microscopy was pioneered and patented by Winfried Denk and James Strickler in the lab of Watt W. Webb at Cornell University in 1990. They combined the idea of two-photon absorption with the use of a laser scanner.[6][7] In two-photon excitation microscopy an infrared laser beam is focused through an objective lens. The Ti-sapphire laser normally used has a pulse width of approximately 100 femtoseconds and a repetition rate of about 80 MHz, allowing the high photon density and flux required for two photons absorption and is tunable across a wide range of wavelengths. Mode-locked Yb-doped fiber lasers with 325 fs pulses have also been employed for collagen imaging, demonstrating a penetration depth of beyond 320 μm in collagen, which is considerably superior to depths of 250 to 300 μm achievable when coupled to a conventional Ti-sapphire excitation laser.
The use of infrared light to excite fluorophores in light-scattering tissue has added benefits.[8] Longer wavelengths are scattered to a lesser degree than shorter ones, which is a benefit to high-resolution imaging. In addition, these lower-energy photons are less likely to cause damage outside the focal volume. Compared to a confocal microscope, photon detection is much more effective since even scattered photons contribute to the usable signal. These benefits for imaging in scattering tissues were only recognized several years after the invention of two-photon excitation microscopy.[9] There are several caveats to using two-photon microscopy: The pulsed lasers needed for two-photon excitation are much more expensive than the continuous wave (CW) lasers used in confocal microscopy. The two-photon absorption spectrum of a molecule may vary significantly from its one-photon counterpart. For very thin objects such as isolated cells, single-photon (confocal) microscopes can produce images with higher optical resolution due to their shorter excitation wavelengths. In scattering tissue, on the other hand, the superior optical sectioning and light detection capabilities of the two-photon microscope result in better performance.
Applications
Two-photon microscopy has been involved with numerous fields including: physiology, neurobiology, embryology and tissue engineering. Even thin, nearly transparent tissues (such as skin cells) have been visualized with clear detail due to this technique.[10] Two-photon microscopy's high speed imaging capabilities may also be utilized in noninvasive optical biopsy.[11] In cell-biology, Two-photon microscopy has been aptly used for producing localized chemical reactions.[9]
Higher-order excitation
Simultaneous absorption of three or more photons is also possible, allowing for Three-photon excitation microscopy.[12]
Dyes for two-photon excitation microscopy
Several green, red and NIR emitting dyes (probes and reactive labels) with high 2-photon absorption cross sections are reported in.[13] Due to the donor-acceptor-donor type structure, squaraine dyes such as Seta-670, Seta-700 and Seta-660 exhibit very high 2-photon absorption (2PA) efficiencies in comparison to other dyes,[13][14][15] SeTau-647 and SeTau-665, a new type of squaraine-rotaxanes, exhibit extremely high two-photon action cross-sections of up to 10,000 GM in the near IR region, unsurpassed by any other class of organic dyes.
SeTau-647: 3,500 GM; λ2P 875 - 925 nm (ε = 200,000 M−1cm−1), λEm = 695 nm (QY = 0.61), τ = 3.2 ns; and some other 2P dyes [13]
See also
- 3D optical data storage
- Nonlinear optics
- Second harmonic imaging microscopy
- Wide-field multiphoton microscopy
References
- ↑ Denk W.; Strickler J.; Webb W. (1990). "Two-photon laser scanning fluorescence microscopy". Science. 248 (4951): 73–6. Bibcode:1990Sci...248...73D. doi:10.1126/science.2321027. PMID 2321027.
- ↑ Goeppert-Mayer M. (1931). "Über Elementarakte mit zwei Quantensprüngen". Annals of Physics. 9 (3): 273–95. Bibcode:1931AnP...401..273G. doi:10.1002/andp.19314010303.
- ↑ Kaiser, W.; Garrett, C. (September 1961). "Two-Photon Excitation in CaF2:Eu2+". Physical Review Letters. 7 (6): 229–231. Bibcode:1961PhRvL...7..229K. doi:10.1103/PhysRevLett.7.229.
- ↑ Abella, I. D. (December 1962). "Optical Double-Photon Absorption in Cesium Vapor". Physical Review Letters. 9 (11): 453–455. Bibcode:1962PhRvL...9..453A. doi:10.1103/PhysRevLett.9.453.
- ↑ Kaminer, Ido; Nemirovsky Jonathan; Segev Mordechai (2013). "Optimizing 3D multiphoton fluorescence microscopy". Optics Letters. 38 (19): 3945–3948. Bibcode:2013OptL...38.3945K. doi:10.1364/OL.38.003945.
- ↑ Denk W.; Strickler J.H.; Webb W.W. (1990). "Two-photon laser scanning fluorescence microscopy". Science. 248 (4951): 73–76. doi:10.1126/science.2321027.
- ↑ US 5034613 "Two-photon laser microscopy."
- ↑ Helmchen F.; Denk W. (2005). "Deep tissue two-photon microscopy". Nat Methods. 2 (12): 932–40. doi:10.1038/nmeth818. PMID 16299478.
- 1 2 Denk W.; Delaney K. (1994). "Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy". J Neurosci Methods. 54 (2): 151–62. doi:10.1016/0165-0270(94)90189-9. PMID 7869748.
- ↑ Masters BR.; So PTC; Gratton E. (1997). "Multiphoton excitation fluorescence microscopy and spectroscopy of in vivo human skin". Biophysical Journal. 72: 2405–2412. Bibcode:1997BpJ....72.2405M. doi:10.1016/s0006-3495(97)78886-6.
- ↑ Bewersdorf J, Rainer P, Hell SW (1998). "Multifocal multiphoton microscopy". Optics Letters. 23: 665–667. Bibcode:1998OptL...23..655B. doi:10.1364/ol.23.000655. PMID 18087301.
- ↑ Xu, Chris; Zipfel, Warren; Shear, Jason B.; Williams, Rebecca M.; Webb, Watt W. (October 1996). "Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy". PNAS. 93 (20): 10763–10768. Bibcode:1996PNAS...9310763X. doi:10.1073/pnas.93.20.10763. PMID 8855254.
- 1 2 3 Podgorski K.; Terpetschnig E.; Klochko O.P.; Obukhova O.M.; Haas K. (2012). "Ultra-Bright and -Stable Red and Near-Infrared Squaraine Fluorophores for In Vivo Two-Photon Imaging". PLOS ONE. 7 (12): e51980. Bibcode:2012PLoSO...751980P. doi:10.1371/journal.pone.0051980. PMC 3522634
 . PMID 23251670.
. PMID 23251670. - ↑ Liu L, et al. (2008). "Homogeneous immunoassay based on two-photon excitation fluorescence resonance energy transfer". Anal. Chem. 80: 7735–7741. doi:10.1021/ac801106w.
- ↑ Przhonska O.V.; et al. (2010). "Two-photon absorption in NIR conjugated molecules: design strategy and structure-property relations in Advance Fluorescence Reporters in Chemistry and Biology (Vol. Ed. Demchenko)". Springer Series of Fluorescence (Series Ed. O.S. Wolfbeis). 8.
External links
- Two-photon suitable dyes
- introduction to multiphoton microscopy
- Acquisition of Multiple Real-Time Images for Laser Scanning Microscopy (Sanderson microscopy article)
- Build Your Own Video-Rate 2-photon Microscope
- Two-photon Fluorescence Light Microscopy, ENCYCLOPEDIA OF LIFE SCIENCES
