Meganeura
| Meganeura Temporal range: 305–299 Ma | |
|---|---|
 | |
| Reconstruction | |
 | |
| Meganeura monyi | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Superorder: | Odonatoptera |
| Order: | †Meganisoptera |
| Family: | †Meganeuridae |
| Genus: | †Meganeura |
| Species | |
| |
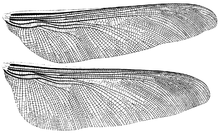
Meganeura is a genus of extinct insects from the Carboniferous period (approximately 300 million years ago), which resembled and are related to the present-day dragonflies. With wingspans of up to 65 cm (25.6 in), M. monyi is one of the largest known flying insect species; the Permian Meganeuropsis permiana is another. Meganeura were predatory, and fed on other insects.
Fossils were discovered in the French Stephanian Coal Measures of Commentry in 1880. In 1885, French paleontologist Charles Brongniart described and named the fossil "Meganeura" (large-nerved), which refers to the network of veins on the insect's wings. Another fine fossil specimen was found in 1979 at Bolsover in Derbyshire. The holotype is housed in the Muséum national d'histoire naturelle, Paris.
Size
There has been some controversy as to how insects of the Carboniferous period were able to grow so large. The way oxygen is diffused through the insect's body via its tracheal breathing system puts an upper limit on body size, which prehistoric insects seem to have well exceeded. It was originally proposed (Harlé & Harlé, 1911) that Meganeura was able to fly only because the atmosphere at that time contained more oxygen than the present 20%. This hypothesis was initially dismissed by fellow scientists, but has found approval more recently through further study into the relationship between gigantism and oxygen availability.[1] If this hypothesis is correct, these insects would have been susceptible to falling oxygen levels and certainly could not survive in our modern atmosphere. Other research indicates that insects really do breathe, with "rapid cycles of tracheal compression and expansion".[2] Recent analysis of the flight energetics of modern insects and birds suggests that both the oxygen levels and air density provide an upper bound on size.[3]
The presence of very large Meganeuridae with wing spans rivaling those of Meganeura during the Permian, when the oxygen content of the atmosphere was already much lower than in the Carboniferous, presented a problem to the oxygen-related explanations in the case of the giant dragonflies. However, despite the fact that meganeurids had the largest known wing spans, their bodies were not very large, being smaller than those of several living Coleoptera; therefore they were not true giant insects, only being giant in comparison with their living relatives. Other explanations for the large size of meganeurids compared to living relatives are warranted.[4] Bechly (2004) suggested that the lack of aerial vertebrate predators allowed pterygote insects to evolve to maximum sizes during the Carboniferous and Permian periods, perhaps accelerated by an evolutionary "arms race" for increase in body size between plant-feeding Palaeodictyoptera and Meganisoptera as their predators.[5] Another theory suggests that insects that developed in water before becoming terrestrial as adults grew bigger as a way to protect themselves against the high levels of oxygen.[6]
References
- ↑ Gauthier Chapelle & Lloyd S. Peck (May 1999). "Polar gigantism dictated by oxygen availability". Nature. 399 (6732): 114–115. doi:10.1038/20099.
Oxygen supply may also have led to insect gigantism in the Carboniferous period, because atmospheric oxygen was 30-35% (ref. 7). The demise of these insects when oxygen content fell indicates that large species may be susceptible to such change. Giant amphipods may therefore be among the first species to disappear if global temperatures are increased or global oxygen levels decline. Being close to the critical MPS limit may be seen as a specialization that makes giant species more prone to extinction over geological time.
- ↑ Westneat MW, Betz O, Blob RW, Fezzaa K, Cooper WJ, Lee WK (January 2003). "Tracheal respiration in insects visualized with synchrotron x-ray imaging". Science. 299 (5606): 558–560. doi:10.1126/science.1078008. PMID 12543973.
Insects are known to exchange respiratory gases in their system of tracheal tubes by using either diffusion or changes in internal pressure that are produced through body motion or hemolymph circulation. However, the inability to see inside living insects has limited our understanding of their respiration mechanisms. We used a synchrotron beam to obtain x-ray videos of living, breathing insects. Beetles, crickets, and ants exhibited rapid cycles of tracheal compression and expansion in the head and thorax. Body movements and hemolymph circulation cannot account for these cycles; therefore, our observations demonstrate a previously unknown mechanism of respiration in insects analogous to the inflation and deflation of vertebrate lungs.
- ↑ Robert Dudley (April 1998). "Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotion performance". The Journal of Experimental Biology. 201 (Pt8): 1043–1050. PMID 9510518.
Uniformitarian approaches to the evolution of terrestrial locomotor physiology and animal flight performance have generally presupposed the constancy of atmospheric composition. Recent geophysical data as well as theoretical models suggest that, to the contrary, both oxygen and carbon dioxide concentrations have changed dramatically during defining periods of metazoan evolution. Hyperoxia in the late Paleozoic atmosphere may have physiologically enhanced the initial evolution of tetrapod locomotor energetics; a concurrently hyperdense atmosphere would have augmented aerodynamic force production in early flying insects. Multiple historical origins of vertebrate flight also correlate temporally with geological periods of increased oxygen concentration and atmospheric density. Arthropod as well as amphibian gigantism appear to have been facilitated by a hyperoxic Carboniferous atmosphere and were subsequently eliminated by a late Permian transition to hypoxia. For extant organisms, the transient, chronic and ontogenetic effects of exposure to hyperoxic gas mixtures are poorly understood relative to contemporary understanding of the physiology of oxygen deprivation. Experimentally, the biomechanical and physiological effects of hyperoxia on animal flight performance can be decoupled through the use of gas mixtures that vary in density and oxygen concentration. Such manipulations permit both paleophysiological simulation of ancestral locomotor performance and an analysis of maximal flight capacity in extant forms.
- ↑ Nel A.N., Fleck G., Garrouste R. and Gand, G. (2008): The Odonatoptera of the Late Permian Lodève Basin (Insecta). Journal of Iberian Geology 34(1): 115-122 PDF
- ↑ Bechly G. (2004): Evolution and systematics. pp. 7-16 in: Hutchins M., Evans A.V., Garrison R.W. and Schlager N. (eds): Grzimek's Animal Life Encyclopedia. 2nd Edition. Volume 3, Insects. 472 pp. Gale Group, Farmington Hills, MI PDF
- ↑ Why Giant Bugs Once Roamed the Earth
External links
![]() Media related to Meganeura at Wikimedia Commons
Media related to Meganeura at Wikimedia Commons
- Picture of life sized model of Meganeura monyi made for Denver Museum of Natural History.